- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Assisted Dying Bill Passed In UK: What It Means And How It Changes For End-of-Life Care Forever?

Credits: AP
The UK Parliament has passed the Terminally Ill Adults (End of Life) Bill in the House of Commons on June 20, 2025, paving the way for potentially the most revolutionary legislation in the country’s approach to end-of-life care regime. The bill, which was narrowly approved by a vote of 314 to 291, permits mentally capable adults 18 years and older, who have been diagnosed with an incurable illness and have fewer than six months to live, to legally ask for medical help to end their life.
The bill, introduced by Labour MP Kim Leadbeater, received cross-party backing after months of fierce debate and modification. It now heads to the House of Lords for further consideration. When passed there, it will align England and Wales with a number of other nations such as Belgium and the Netherlands which have legalized assisted dying under strict conditions.
Terminally Ill Adults (End of Life) Bill sets out a thorough procedure to guarantee both safety and freedom. A patient applying for assisted dying must be:
- Diagnosed with a terminal condition and have a prognosis of less than six months
- Mentally capable of making decisions
- A permanent resident in England or Wales and have been registered with a GP for at least 12 months
- Making a settled, informed, and clear decision that is not under coercion
Two independent physicians shall verify the diagnosis and the mental capacity of the patient. An expert multidisciplinary panel, in lieu of the initially proposed function of a high court judge, will then consider the request. It consists of a legal expert, psychiatrist, social worker, and medical professionals.
What Will The Process Be Like?
The bill also features a two-stage declaration procedure. Two declarations, signed and witnessed, are required to be made by patients to confirm their decision. Even with panel approval, there is a required 14-day cooling-off period unless death is imminent.
The most important protection: the life-ending drug has to be given by the patient to themselves. Nobody—no doctor, no relative—can legally administer the dose to the patient. This provision meets ethical objections and reduces coercion risks.
Also, every kind of pressure or coercion is a criminal act according to the bill and it can lead to up to 14 years in prison. This is a good deterrent against any kind of misuse of law.
What Happens Next Before Implementation?
While the bill has passed the House of Commons, it still needs to clear all stages in the House of Lords. After Lords' scrutiny and any proposed changes, the bill returns to the Commons for final approval. Only then can it receive Royal Assent and become law.
If passed, implementation could take up to four years, delaying the first possible legal assisted deaths to as far as 2029. The timeframe allows for infrastructural requirements, setting up the expert panels, and public health preparedness.
The four-year window is a "backstop" rather than an absolute deadline. Leadbeater's office says the process may be speeded up based on legislative and logistical developments.
The Assisted Dying Bill has evoked passionate views throughout the UK. A Royal College of Surgeons 2023 survey revealed 53% in favor of assisted dying and 25% against. A comparable BMA survey in 2020 revealed a 50% rate in favor and 39% against.
These statistics portray a complex debate among doctors. Acknowledging this, the bill has a conscience clause permitting medical professionals to withhold participation in the process.
Until recently, assisted suicide was prohibited in England, Wales, and Northern Ireland, with a penalty of up to 14 years' imprisonment. The new bill represents a seismic change in how terminally ill patients can meet their demise, shifting end-of-life care from an exclusively palliative approach to a model incorporating medically assisted dying.
An impact assessment by the government estimates that between 160 and 640 people may opt for assisted deaths in the bill's first year, potentially rising to 4,500 annually over the next decade. These projections highlight the potential scale of the law's impact on healthcare systems, ethical practices, and family dynamics.
Will The Bill Learn from International Models?
Belgium, the Netherlands, and Canada have all provided precedents in assisted dying, frequently referenced by advocates of the UK bill. These countries have established robust regulatory systems, multidisciplinary assessment, and strict consent measures—all features reflected in the UK's proposed legislation.
Yet, every nation's experience has also been a warning. The UK bill takes note of lessons from overseas, especially on protecting against involuntary euthanasia and ensuring that mental illness on its own does not qualify for assisted dying.
As the UK Parliament pushes on, the rest of the UK is developing its own assisted dying laws. The Scottish Parliament debated a similar bill at the same time, and in March 2025, the Isle of Man became the first British jurisdiction to pass such legislation.
This decentralized tactic enables regional adaptation and underscores the increasing public call for independence in end-of-life care throughout the UK.
What is the Ethical and Philosophical Controversy Around?
For supporters, the bill is about dignity, autonomy, and freedom from unnecessary suffering. For detractors, it speaks to possible abuses, the moral load placed on medical professionals, and the message it conveys to society about the worth of life.
Nevertheless, with Parliament making a definitive step ahead, the controversy now shifts to a new level—one based not only on ideology, but on implementation.
The UK's Assisted Dying Bill is not only a bill; it is a redefinition of terminal care compassion. As it makes its way through the House of Lords, the country teeters on the threshold of a historic shift in its treatment of the dying. If enacted into law, the effect could be sweeping—providing terminally ill patients with the right to a dignified, self-controlled death, while redefining ethical, medical, and legal paradigms for future generations to come.
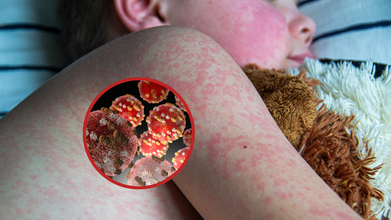
Measles Cases Surge Worldwide, Korean Health Data Flags Countries At Highest Risk For Travellers
(Credit-Canva)
Health officials around the world are warning travelers about a sharp increase in measles cases. The Korea Disease Control and Prevention Agency (KDCA) is urging everyone to be fully vaccinated before traveling abroad, as many recent cases in Korea have been from travelers returning from other countries.
Globally, measles cases have surged, with the World Health Organization (WHO) reporting around 360,000 cases in 2024. This is largely because of a drop in vaccination rates, which were disrupted during the COVID-19 pandemic
What Should Travellers Be Aware Of?
According to the KDCA, many of these infections came from countries with ongoing measles outbreaks. The number of global measles cases jumped to about 360,000 in 2024, as vaccination efforts were disrupted by the COVID-19 pandemic. The countries linked to the most imported cases are:
- Vietnam
- South Africa
- Uzbekistan
- Thailand
- Italy
- Mongolia
For travelers heading to these regions, the KDCA strongly recommends getting both doses of the measles, mumps, and rubella (MMR) vaccine. This is especially important for adults who may have missed their second dose as children.
Countries That Have The Highest Measles Cases
According to a Pan American Health Organization (PAHO) report released on 15th August, there have been 10,139 confirmed cases and 18 deaths across ten countries, a 34-fold increase compared to the same period in 2024. The countries with the highest number of confirmed cases are:
- Canada (4,548 cases)
- Mexico (3,911 cases)
- United States (1,356 cases)
Cases have also been reported in Bolivia, Argentina, Belize, Brazil, Paraguay, Peru, and Costa Rica. Sadly, there have been deaths in Mexico (14), the U.S. (3), and Canada (1). In Mexico, most of the deaths happened in Indigenous communities, which have been severely affected. Canada also reported the tragic death of a newborn.
The outbreaks are being caused by two different types of the measles virus. One type is spreading quickly among Mennonite communities in eight countries. This shows how fast the virus can move through groups that are not vaccinated. While the outbreaks have mainly been in these communities, more and more cases are now being found in other groups of people as well..
Why Do You Need Measles Vaccine?
Measles is a very contagious illness that can spread easily through the air. While most people think of it as a childhood disease, it can cause serious problems like pneumonia or brain swelling. It's especially dangerous for young children, pregnant women, and people with weak immune systems.
The MMR vaccine is the best way to protect yourself and others. Officials strongly recommend getting both doses, especially if you plan to travel. The KDCA also advises anyone who develops a fever or rash within three weeks of returning to Korea to take immediate precautions: wear a mask, limit contact with others, and tell medical staff about your recent travel before seeking care.
What Steps Have The Countries With Measles Outbreak Taken?
According to PAHO,
- Measles is still spreading in several provinces of Canada, including Alberta and British Columbia.
- In Mexico, the government has launched a mass vaccination campaign in 14 high-priority areas, with a special focus on Indigenous communities in Chihuahua, where most of the country's cases are located.
- In United States, outbreaks have been reported in 41 states, mainly among under-vaccinated populations.
- Most cases of Bolivia are in the Santa Cruz area, affecting both the general population and Mennonite communities.
- Argentina and Belize have not reported any new cases since late June.
PAHO is working with these countries to boost vaccination rates and quickly respond to new outbreaks. While travel restrictions aren't recommended, travelers are advised to ensure they are vaccinated, including young children from 6 to 11 months old, who can get an early dose for protection.
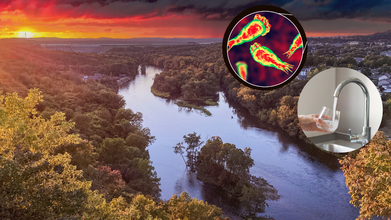
Missouri Resident Hospitalized With 97% Fatal 'Brain-Eating' Infection While Water-skiing: How Real Is The Risk At Home?
Credits: Health and me
A Missouri resident is fighting for life in the intensive care unit after contracting one of the rarest yet most deadly infections known to medicine: Naegleria fowleri, often referred to as the brain-eating amoeba. The case, traced back to waterskiing on the popular Lake of the Ozarks, has sparked renewed questions about how people can be exposed to this microscopic threat—and whether everyday activities like showering or drinking tap water could carry risks.
Naegleria fowleri is a single-celled organism that lives naturally in warm freshwater such as lakes, rivers, and hot springs. It thrives in temperatures between 77°F and 115°F, meaning it is more commonly found in waters during hot summer months when levels are low and temperatures rise.
Despite its frightening nickname, the amoeba doesn’t “eat” brains in a literal sense. Instead, when water contaminated with N. fowleri enters through the nose—often during swimming, diving, or watersports—the organism can travel to the brain. Once there, it causes primary amebic meningoencephalitis (PAM), a devastating infection that destroys brain tissue.
The U.S. Centers for Disease Control and Prevention (CDC) describes PAM as “almost always fatal.” In fact, the fatality rate exceeds 97%, with just four survivors out of 167 confirmed cases in the United States between 1962 and 2024.
How Does the Infection ?
The key factor is nasal exposure, not ingestion. Simply drinking contaminated water does not cause illness. The amoeba must enter through the nose and reach the brain’s olfactory nerves. This is why activities like:
- Waterskiing, wakeboarding, or diving in warm freshwater
- Swimming in poorly maintained pools or splash pads
- Using contaminated tap water for nasal rinses or Neti pots
are considered riskier than simply consuming the water.
Cases have even been linked to routine activities such as bathing or showering in places where water systems were contaminated. While rare, this route of exposure is considered possible if water is forced high into the nasal cavity.
What Are the Symptoms?
Symptoms usually develop within 3 to 12 days after exposure. They begin subtly, resembling common viral illnesses, but progress rapidly:
Early: headache, fever, nausea, vomiting
Later: stiff neck, confusion, hallucinations, seizures
Advanced: coma and death, typically within five days of symptom onset
This rapid progression makes timely diagnosis extraordinarily difficult. By the time PAM is suspected, the infection is usually advanced.
How Rare Is Brain-Eating Infection, Really?
The good news is that Naegleria fowleri infections are extremely rare. In the U.S., an average of just two to three cases per year are reported, despite millions of people swimming in warm lakes and rivers.
Globally, however, cases have been documented in more than 40 countries. A review up to 2018 found 381 cases worldwide, with a shocking 92% mortality rate. The highest number of cases were recorded in the United States, Pakistan, Mexico, and India. Australia, too, has documented outbreaks, particularly in regions with warmer climates.
Is It Safe To Drink Tap-Water?
One of the most unnerving aspects of Naegleria fowleri is when it shows up in municipal water supplies. In 2024, officials in Queensland, Australia, confirmed its detection in drinking water systems in the towns of Augathella and Charleville. So should Americans be worried about catching the infection from their tap? Here’s the science:
Drinking contaminated water does not cause infection. The digestive system neutralizes the amoeba.
Nasal exposure is the risk. Taking a shower, using a Neti pot, or even children playing with hoses or sprinklers could theoretically force water up the nose.
Proper water treatment works. Adequate chlorination and maintenance of municipal systems eliminate the risk.
In the U.S., isolated incidents linked to Neti pots used with unboiled tap water have been reported, underlining why the CDC stresses that nasal rinses must use distilled, sterile, or previously boiled water.
In Missouri, the patient’s exposure was likely tied to high-speed watersports on Lake of the Ozarks during a spell of hot weather. Waterskiing increases the risk because it forces water deeply into the nasal passages.
Officials have not reported other cases connected to the lake, emphasizing just how rare the infection is, even in favorable conditions. Still, the case has heightened awareness and anxiety, particularly as climate change increases the likelihood of warmer water temperatures in many regions.
How Can You Protect Yourself?
There is no guaranteed way to eliminate risk, but health experts offer several practical precautions:
- Avoid swimming in warm freshwater during heatwaves or when water levels are low.
- Keep your head above water in lakes, rivers, or hot springs.
- Don’t dive or jump in. Water forced up the nose is the main risk factor.
- Use nose clips if you plan to submerge in freshwater.
- Stick to saltwater or properly chlorinated pools. The amoeba cannot survive in these environments.
- Use sterile or boiled water for nasal rinses. Never rely on straight tap water.
The idea of a “brain-eating amoeba” is terrifying, but public health experts emphasize that the odds of infection are extraordinarily low. Millions of people swim in lakes every year without incident.
The far greater concern, they say, is that when cases do occur, they are often misdiagnosed as bacterial meningitis until it’s too late. Public awareness, while important, should be balanced with the reality that preventive measures—like avoiding nasal exposure to untreated warm freshwater—are usually enough.
The Missouri case is a tragic reminder of just how deadly Naegleria fowleri can be, but also how vanishingly rare it is. You cannot catch it by drinking tap water, and routine showers in well-maintained municipal systems are safe. The risk only arises when contaminated warm freshwater is forced into the nose.
For now, experts say vigilance, not panic, is the right response. With simple precautions, Americans can keep enjoying summer lakes and rivers while understanding the small but serious risks that come with them.
Flu Nasal Sprays Now Available For At-Home Delivery: Know Risks, Precautions And Side Effects

(Credit-Canva, FluMist)
As harmless as it may seem, flu has been classified as high severity in 2024-25 across all ages, according to the National Foundation for Infectious Diseases (NFID). Amidst the rising number of cases, we may have an easy at-home way to deal with it.
AstraZeneca has launched FluMist Home, a new service that delivers the FLUMIST nasal spray flu vaccine right to your house. This is a game-changer, as it's the first time an influenza vaccine can be sent directly to customers for at-home use.
FLUMIST, a needle-free nasal spray, has been approved by the FDA since 2003. Now, it is also the first flu vaccine approved for people ages 18-49 to give to themselves, or for parents and caregivers to give to children ages 2-17. This new service aims to make it easier for people to get vaccinated and stay protected, especially after the last flu season was one of the worst in years.
Will This At-Home Service Help Lower Flu Cases?
According to NFID, 47 million flu related, 21 million medical visits, 610,000 hospitalizations and 27,000 deaths, which include 266 pediatric deaths happened during the flu season of 2024-25.
AstraZeneca and healthcare experts believe this new service will help more people get vaccinated. It makes flu shots more convenient and removes the need to go to a doctor's office or pharmacy.

Who Should Not Take the Flu Nasal Spray?
According to the FluMist Safety Information available on their site, you should not get the FLUMIST vaccine if any of the following apply to you:
- You have a severe allergy to the vaccine's ingredients, to eggs, or to other flu vaccines.
- You are a child or adolescent between 2 and 17 years old and are currently taking aspirin or aspirin-containing medicines. Children in this age group should not be given aspirin for four weeks after getting FLUMIST unless a doctor says it's okay.
- You are under 2 years old, as there's a higher risk of wheezing (trouble breathing) in this age group after getting the vaccine.
What Precautions Do You Need For the Flu Nasal Spray?
For the 2025-26 flu season, FluMist Home is available in 34 states. AstraZeneca plans to expand the service to all 48 states in the future. FLUMIST will still be available at doctors' offices and pharmacies. Before getting FLUMIST, it's crucial to tell your healthcare provider about all your medical conditions. This includes if you:
- Are currently wheezing or have a history of wheezing (especially if you're under 5 years old).
- Have asthma.
- Have a weakened immune system or live with someone who has a severely weakened immune system.
- Have had Guillain-Barré syndrome (a condition that causes severe muscle weakness).
- Have problems with your heart, kidneys, or lungs.
- Have diabetes.
- Are pregnant or nursing.
- Are taking antiviral drugs to treat the flu.
What Are The Side-Effects of The Flu Nasal Spray?
While most side effects are mild, FLUMIST can cause rare but serious reactions. The most common side effects are a runny or stuffy nose, sore throat, and a fever over 100°F.
Rare, serious side effects can include allergic reactions. If you experience hives, swelling of your face, lips, eyes, tongue, or throat, or have trouble breathing, seek medical help immediately.
© 2024 Bennett, Coleman & Company Limited

