- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Can You Take Antibiotics For Covid-19? Here Is What The WHO Says Now

Back in 2020, the world went on an involuntary vacation. Borders shut, masks went up, and hand sanitiser became the new perfume. Fast forward five years, and Covid-19 is still lurking about and as the virus continues with newer variants like Stratus, currently rising in the US, a familiar question keeps popping up: Can I take antibiotics for Covid? The World Health Organization (WHO) has an answer.
New WHO Guidelines Say “No” to Unnecessary Antibiotics
The WHO has made it clear that antibiotics are not your Covid cure. Even if you are lying in bed with a fever, unless there is actual proof of a bacterial infection, antibiotics should not be part of your recovery plan. According to the updated guidance, even patients with severe Covid-19 should not be given antibiotics by default. The only exception is when there is genuine clinical suspicion of a concurrent bacterial infection. Otherwise, it is a waste and potentially harmful.“For patients with non-severe COVID-19 and a low clinical suspicion of a concurrent bacterial infection, we recommend no empirical antibiotics,” the WHO said.
Why This Change Now?
When Covid-19 first hit the world, doctors prescribed a lot of medicines, including antibiotics. But over time, researchers have wondered if all those pills are doing anything. A recent meta-analysis reviewed patient outcomes and found that antibiotics did not make a difference in people who did not have a bacterial infection.But this is not just about ineffective treatment. It is about antimicrobial resistance.
What Happens When There is Antibiotic Overuse?
When we misuse antibiotics, meaning taking them when we do not need to or not finishing a proper course, we help bacteria evolve into superbugs. These resistant bacteria are tough and do not cure with your average antibiotics. Antimicrobial resistance is already a global health crisis. By needlessly tossing antibiotics at Covid-19, we are just speeding up the problem.What Should You Do If You Get Covid Now?
If you test positive and your symptoms are mild, your best bet is rest, fluids, paracetamol for fever, and patience. Pop the antibiotics only when your doctor prescribes. Also, Covid is now handled like any other respiratory illness. That is why WHO has also scrapped older Covid-specific guidelines that no longer apply to the post-pandemic world.“Notable changes to COVID-19 disease over this time have been overall reduced infection rates and reduced disease severity,” the WHO said, adding that "Care for patients with COVID-19 has become more integrated with usual healthcare systems.”
In a nutshell, you cannot take antibiotics for Covid unless you also have a bacterial infection. And that is for a healthcare professional to decide. The WHO wants everyone, from doctors and pharmacists to patients and panic-buyers, to get on the same page. Antibiotics are powerful tools, and using them wisely could literally save the world.
However, the global health body warned that the virus continues to evolve in terms of infectivity, immune escape, and disease severity. “This guideline robustly and transparently addresses the changing landscape and
evidence availability, and the continual development of treatment and management strategies for Covid-19,” the WHO added.
Eli Lilly’s New Weight Loss Pill Helped People With Obesity Shed Nearly 15% In Late-Stage Trials

Credits: Canva
Eli Lilly’s experimental weight loss pill, orforglipron, may soon shift the landscape of obesity treatment. In a Phase 3 clinical trial that spanned more than 3,000 adults, the once-daily pill helped participants lose an average of 12.4% of their body weight over 72 weeks—with minimal lifestyle restrictions and no needles involved.
The company says these findings mark a turning point in the battle against obesity, one of the most pressing global health issues today. Here’s how this oral medication is poised to change everything we know about weight loss treatment.
According to Eli Lilly’s preliminary data, adults who took the highest dose of orforglipron lost an average of 27.3 pounds (12.4% of their body weight) over roughly a year and a half. Nearly 60% of those participants lost at least 10% of their weight, and almost 40% lost at least 15%.
These results are on par with injectable GLP-1 medications like Zepbound and Mounjaro—both also made by Eli Lilly—which have become blockbuster drugs in the obesity and diabetes market.
Dr. Céline Gounder, editor-at-large for public health at KFF Health News, put it bluntly in a CBS interview: “This is what we see with injectables. It’s impressive that a pill could match that.”
The key distinction? Orforglipron is not a peptide-based medication. That makes it easier for the body to absorb and eliminates the need for the strict dietary rules that apply to oral peptide drugs like Novo Nordisk’s Rybelsus.
How GLP-1 in Pill Form Works?
Orforglipron belongs to a class of medications called GLP-1 receptor agonists. These drugs mimic a gut hormone that plays a critical role in appetite regulation and glucose metabolism. They slow down stomach emptying, curb hunger signals in the brain, and help stabilize blood sugar levels.
Until recently, the most effective GLP-1 medications had to be injected, often weekly. Eli Lilly’s innovation—compressing this powerful mechanism into an oral pill—is a major step forward.
Unlike Rybelsus, the only approved GLP-1 pill on the market (which requires fasting and water restrictions), orforglipron is designed to be taken at any time of day, with or without food or water. That alone could make a big difference for patient compliance and everyday ease of use.
Are There Significant Side Effects For This Pill?
The benefits are clear, but what about the risks? As with other GLP-1-based drugs, gastrointestinal side effects were common. The most reported issues included nausea, diarrhea, constipation, and vomiting. Between 29% and 34% of participants experienced nausea—slightly higher than the 25–29% range seen with injectable Zepbound.
More significantly, about 10% of those on the highest dose had to drop out of the study due to side effects. While not uncommon in drug trials, it’s a notable figure for clinicians to weigh when considering patient suitability.
Still, experts say the side effect profile remains consistent with what is expected from GLP-1 medications. Importantly, orforglipron was also associated with improved markers of cardiovascular health, such as lower cholesterol and blood pressure.
What Makes Orforglipron Stand Out?
One of the most promising aspects of this pill is its potential to support long-term weight maintenance. Eli Lilly is also studying orforglipron’s role in helping people who initially lose weight on injectable drugs but are looking for a more manageable, sustainable option moving forward.
In essence, this pill could serve as a bridge—or even an off-ramp—for those who can’t or don’t want to stay on injectable medications forever. The convenience factor is huge.
There’s also the question of cost. Manufacturing pills is typically cheaper than producing injectables, and while pricing hasn’t been announced yet, orforglipron could offer a more affordable option for millions of people who need access to effective obesity care.
Eli Lilly has announced plans to submit orforglipron for regulatory approval by the end of 2025, with hopes of a global launch to follow soon after. If approved by the FDA and other agencies, the drug could hit the market in 2026.
This timeline is aggressive but realistic, given the urgency of the obesity crisis. More than 40% of American adults are classified as obese, according to CDC data. The economic burden and health consequences—including increased risk of heart disease, stroke, diabetes, and certain cancers—are staggering.
The public health need is urgent, and the demand for safe, convenient, and effective weight-loss treatments is at an all-time high. A pill like orforglipron, if approved, could help close that gap.
Despite the promising clinical data, not everyone is convinced just yet. Eli Lilly’s stock fell nearly 14% on the day of the announcement. Some investors expected even greater weight-loss figures or a better side effect profile.
But experts caution against reading too much into the stock market’s knee-jerk reactions. The potential of a widely accessible, non-peptide, once-daily GLP-1 pill is enormous—and it could spark a new era in weight management.
If successful, orforglipron will likely intensify the competition with Novo Nordisk, whose Wegovy and Ozempic have dominated the obesity and diabetes space for the past few years. The race is now on for the first truly scalable, cost-effective oral GLP-1 option—and Lilly may have just edged ahead.
Eli Lilly’s orforglipron isn’t just another weight-loss drug. It’s a potential paradigm shift in how we approach chronic weight management.
If it clears the final regulatory hurdles and delivers on its early promise, orforglipron could bring highly effective, user-friendly weight-loss treatment to millions of people—no needles, no meal timing, no fuss. That’s the kind of change the obesity crisis has been waiting for.
Could Lithium Deficiency In The Brain Trigger Alzheimer’s?
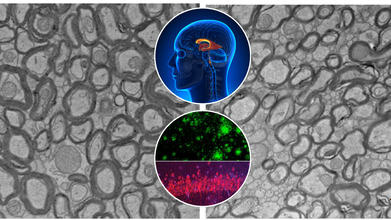
Credits: Yankner Lab/Harvard Medical School
Alzheimer's disease has confounded scientists and destroyed millions of families globally. While advances have been made toward grasping the disease's biological markers—amyloid plaques, tau tangles, and neuroinflammation therapies have generally fallen far short of significant reversal or prevention. A recent study at Harvard Medical School now indicates something remarkable: a natural trace element lithium could be an overlooked piece to the Alzheimer's puzzle.
The study, which appeared in Nature, identifies that lithium is not only a psychiatric medication. It occurs naturally in the human brain and could be necessary for shielding brain cells from aging and degeneration. In mice, the lack of lithium hastened the development and severity of Alzheimer's-like symptoms. More importantly, restoring lithium—specifically in a form that avoids getting trapped by amyloid beta reversed brain damage and brought back lost memory.
What this really means is that lithium isn’t just a potential treatment. It could help explain why Alzheimer’s develops in the first place.
Alzheimer’s doesn’t affect everyone equally. Some people accumulate classic markers like amyloid plaques or tau tangles in their brains but never develop dementia. Others experience sharp cognitive decline despite only mild physical changes.
Dr. Bruce Yankner and his team at Harvard’s Blavatnik Institute believe that lithium levels may be part of the answer. Their ten-year study shows that natural lithium in the brain—like other vital nutrients such as iron or vitamin C—plays a central role in protecting neurons. When levels drop, the shield weakens, leaving the brain vulnerable to damage.
Using advanced spectroscopy on postmortem brain tissue from thousands of individuals, the researchers found that lithium levels were high in cognitively healthy people but markedly reduced in those with mild cognitive impairment or Alzheimer’s. These changes appeared before significant brain damage had occurred, suggesting lithium depletion might kickstart the disease—not just accompany it.
Lithium is well-known in psychiatric medicine. It's a gold-standard treatment for bipolar disorder and is also used in some forms of depression and schizophrenia. At high doses, it stabilizes mood by modulating brain chemicals like serotonin. But its therapeutic window is narrow: too much can lead to kidney or thyroid issues, especially in older adults.
What’s different here is the discovery that tiny, natural levels of lithium—one-thousandth the dose used in psychiatry—may be enough to maintain healthy brain function and prevent neurodegeneration.
“It’s the first time anyone’s shown that lithium exists at a biologically meaningful level in the brain, without administering it as a drug,” Yankner explains. “That’s a game-changer.”
The study used Alzheimer’s-prone mice to examine how lithium levels influence disease progression. When fed lithium-deficient diets, these mice developed early-onset symptoms brain inflammation, memory loss, and accelerated aging. Their brains showed more amyloid plaques, impaired microglial activity, and damage to neuron-protecting myelin.
Conversely, when researchers gave the mice a novel compound called lithium orotate—a version of lithium that bypasses the traps laid by amyloid beta—the mice not only stabilized, they reversed their cognitive decline. In some cases, older mice regained previously lost memories.
The compound worked at ultra-low doses and caused no observable toxicity, even when administered over the animals’ entire lifespans.
Why Lithium Gets Trapped in the Brain?
One of the most surprising discoveries was that amyloid beta binds to lithium, sequestering it and preventing it from doing its job. This happens early in the disease process, even before symptoms arise.
By identifying lithium compounds that evade this trap, researchers opened the door to a new treatment path. Lithium orotate appears to preserve lithium's benefits without falling victim to amyloid beta interference—something that traditional compounds like lithium carbonate fail to do.
This finding could explain why previous clinical trials using conventional lithium compounds in Alzheimer’s have had mixed results or were abandoned due to side effects.
Can Lithium Prevent Alzheimer’s?
This new perspective brings up a big question- could lithium be used not just to treat, but prevent Alzheimer’s? Yankner and his colleagues believe so.
Their findings align with earlier population studies showing lower dementia rates in areas with naturally higher lithium levels in drinking water. The new study, however, goes further. It not only confirms that lithium is present in the brain naturally, but also shows what happens when it's lost—and how replenishing it can make a difference.
This opens the door to new screening methods. If lithium levels can be measured through a simple blood test, they could become a biomarker for early Alzheimer’s risk, much like cholesterol for heart disease.
What Is Lithium Used For?
Lithium is a naturally occurring trace mineral found in soil, water, and certain foods. While it's most widely known as a psychiatric medication—used in higher pharmaceutical doses to treat bipolar disorder and stabilize mood—its nutritional role is lesser known. In micro amounts, lithium appears to influence brain health, cognitive function, and emotional regulation. Some researchers suggest it may have neuroprotective properties, helping to shield the brain from age-related decline, though more data is needed.
What Happens When You Lithium Deficiency?
Although there’s no officially recognized “lithium deficiency” like there is for iron or calcium, emerging research hints that extremely low levels of lithium intake may be associated with higher rates of mood disorders, neurodegenerative conditions, and even increased mortality. Some ecological studies have shown that populations consuming higher natural levels of lithium in drinking water report lower suicide rates and better mental health outcomes. This doesn't mean everyone should start supplementing with lithium, but it does raise important questions about its role in overall neurological and psychological well-being.
Are Human Trials Next?
While the findings in mice are compelling, the leap to human treatment requires rigorous clinical trials. Lithium orotate has not yet been tested in Alzheimer’s patients, and its long-term safety in this context remains unproven.
Still, the research offers a rare blend of explanatory power and therapeutic promise—something that’s often lacking in Alzheimer’s science.
“If this pans out in human trials, it could fundamentally shift how we detect, prevent, and treat Alzheimer’s,” Yankner says. “It’s the most far-reaching effect I’ve ever seen from a single compound.” But he also urges caution. People should not self-medicate with lithium. Over-the-counter lithium supplements are unregulated and could be dangerous, especially at the wrong dose or in older adults with kidney conditions.
This isn’t just another Alzheimer’s study focused on a single protein or pathway. It proposes a unifying mechanism—a missing nutrient that may drive the disease and also offer a way back.
Lithium, once viewed solely as a psychiatric drug, may turn out to be something much more profound: a fundamental player in brain health, a natural buffer against degeneration, and perhaps a key to one of the most confounding diseases of our time.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Do not begin lithium supplementation without speaking to a qualified healthcare provider.
‘Lucky To Be Alive', US Woman Wakes Up From A Coma Just Seconds Before Doctors Begin Organ Harvesting
Credits: Canva (representational)
In a case that raises urgent ethical, medical, and systemic questions, a woman in New Mexico, US narrowly escaped having her organs harvested while she was still ALIVE. Her case exposes disturbing gaps in the US organ donation system and has sparked a nationwide debate about the protocols followed when a patient is declared beyond recovery.
Danella Gallegos, 38, was homeless when she suffered a still-unnamed medical crisis in 2022. She fell into a coma and was admitted to Presbyterian Hospital in Albuquerque, New Mexico. There, doctors told her family that her condition was irreversible and that she would never regain consciousness.
With no apparent hope left, the family made the heartbreaking decision to donate her organs to help others in need. In stepped New Mexico Donor Services, the organ procurement organization (OPO) designated to coordinate the logistics of organ harvesting but in the days leading up to the planned surgery, something didn’t feel right to Gallegos’ family.
According to her family, Danella showed subtle but unmistakable signs of life. Her sisters said they noticed tears running down her cheeks — a deeply human response. When they brought this to the donation coordinator's attention, they were told it was nothing more than a reflex.
On the day of the scheduled operation, one of her sisters claimed she felt Danella move when she held her hand. That alone should have raised immediate red flags. But the real shock came when medical staff observed Gallegos blinking in response to verbal commands — an act that clearly suggested consciousness and awareness.
Despite these signs, the organ donation coordinator present in the operating room allegedly encouraged doctors to go forward with the procedure, recommending they administer morphine and complete the surgery.
Against pressure from the donation organization, the physicians canceled the operation. That move saved Danella Gallegos’ life. In time, she emerged from her coma and ultimately made a full recovery.
Speaking after the ordeal, Gallegos admitted she felt fear while in the coma but has only patchy memories of the experience. “I feel so fortunate,” she said. “But it’s also crazy to think how close things came to ending differently.”
In response to growing scrutiny, New Mexico Donor Services denied any wrongdoing. The organization claimed it does not interfere in clinical decision-making and emphasized that only hospitals are responsible for patient care.
Presbyterian Hospital, however, painted a different picture. They asserted that New Mexico Donor Services oversees “all aspects” of the donation process. The hospital has since launched an investigation into Gallegos’s case.
Neva Williams, a veteran intensive care nurse at the hospital, offered a chilling summary to The New York Times: “All they care about is getting organs. They’re so aggressive. It’s sickening.”
This back-and-forth has laid bare the uncomfortable tension between medical ethics and the demand for transplantable organs. Here’s where it gets complicated, more than 103,000 people in the U.S. are currently on organ transplant waiting lists. Every day, approximately 13 people die waiting.
Organ procurement organizations exist to speed up the donation process and match recipients with available organs. Each donor can potentially save up to eight lives and improve 75 more through tissue and corneal donations.
Because viable organs have a very short shelf life after death, timing is everything. That urgency can create pressure — sometimes overwhelming — on hospitals and families.
The most ethically thorny donations happen under the classification of donation after circulatory death (DCD). Unlike brain death, where all brain activity has irreversibly ceased, patients in a DCD state may still have limited brain function and are typically removed from life support before organ removal begins. Doctors must wait until the heart has stopped beating for at least five minutes before they can begin the transplant process — otherwise, the organ is no longer viable.
In 2024, these DCD cases made up about one-third of all organ donations, according to government data.
What Are The Organ Donation Ethics Globally?
The United Kingdom uses an opt-out system for organ donation — meaning adults are presumed to be donors unless they explicitly say otherwise. Still, the legal framework places strong emphasis on confirming death through strict medical criteria before any organs are taken. Two main types of death are recognized:
- Circulatory death, where the heart stops beating for five continuous minutes.
- Brain stem death, which ensures the individual will never regain consciousness or breathe independently.
Importantly, in the UK, donation coordinators are strictly barred from intervening in any decisions related to life support withdrawal or patient care.
Danella Gallegos’ case has reignited an uncomfortable but necessary conversation: How much pressure is too much? When is a patient truly beyond recovery and who gets to decide? It also casts a spotlight on the increasing corporatization of organ donation, where nonprofits are under growing pressure to deliver results — often quantified in organ procurement rates — not human stories.
As the U.S. continues to rely heavily on OPOs to bridge the gap between donors and recipients, oversight and ethical accountability remain patchy at best.
While most organ donation cases proceed ethically and save countless lives, the outliers like Gallegos remind us that getting it wrong isn’t just a procedural misstep. It’s a near-death experience. For now, Gallegos is alive and healing but her experience stands as a stark reminder of what’s at stake when assumptions are made about comatose patients and when time-sensitive procedures begin to edge into dangerous ethical territory.
As hospitals and OPOs continue to work in high-pressure environments, there’s a pressing need for standardized safeguards, better oversight, and a renewed focus on what should always be the top priority, the living patient’s right to be heard, even in silence.
© 2024 Bennett, Coleman & Company Limited

