- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Can Down Syndrome Symptoms Be Reversed?

Credit: Canva
In a significant scientific breakthrough, researchers have successfully reversed memory and learning deficits in a mouse model of Down syndrome (DS) by targeting the body’s response to cellular stress. The findings, published in Science on November 14, 2019, offer new hope for improving cognitive function in individuals with DS — the most common genetic cause of intellectual disability.
What Is The Research About?
A team led by researchers at UC San Francisco and Baylor College of Medicine found that in the brains of DS-model mice, learning impairments were linked to disrupted protein production in the hippocampus — a key brain region responsible for learning and memory. But by using specific drugs that modulate the body’s stress response, they were able to restore protein synthesis and significantly improve cognitive performance.Traditionally, DS research has focused on how an extra copy of chromosome 21 affects gene expression. However, this study took a different route. The scientists zeroed in on "proteostasis," the process by which cells maintain a healthy balance of protein production and regulation. They discovered that in DS mice, the integrated stress response (ISR) — a natural protective mechanism that reduces protein production during cellular stress — was unusually active in hippocampal cells. This reduction in protein synthesis, while generally protective, was impairing the brain’s ability to form long-term memories.
“The cell is always checking in on its own health,” said Dr. Peter Walter, professor of biochemistry at UCSF and co-senior author of the study. “When something’s off, like an extra chromosome, it can trigger a stress response that shuts down protein production — and that’s detrimental for cognitive function.”
Using three different methods, including a drug called ISRIB, the researchers suppressed this stress response and observed dramatic improvements in learning and memory among DS mice. The animals performed significantly better in two behavioural tests and showed enhanced synaptic activity in the brain, suggesting real physiological change.
To strengthen their findings, the team examined brain tissue from people with DS. They found that the ISR was similarly activated in those with the extra chromosome — reinforcing the link between the stress response and DS symptoms.
Interestingly, they identified a specific enzyme, PKR, as the main culprit behind this stress activation. By blocking PKR, either genetically or pharmacologically, the team was able to prevent the decline in protein production and restore cognitive function in mice.
Although these results are preliminary and require further investigation in human models, the study marks a promising first step toward developing treatments for DS. “We started with a situation that looked hopeless,” Walter said. “Nobody thought anything could be done. But we may have struck gold.”
The study was supported by the NIH and the Howard Hughes Medical Institute. While some of the researchers have industry ties related to the drug ISRIB, they report no additional conflicts of interest.
This research signals a paradigm shift — from solely genetic approaches to exploring how cellular stress management could hold the key to treating cognitive disorders like Down syndrome.
Legionnaires’ Outbreak: Norwegian Cruise Line Probes Two Cases Linked to December Encore Sailing
Representational Image (Canva)
Norwegian Cruise Line has notified passengers about two confirmed cases of Legionnaires’ disease linked to a December 2025 sailing aboard its ship, the Norwegian Encore.
In a letter dated February 12 and addressed to recent guests, the cruise operator said it is working with the Centers for Disease Control and Prevention (CDC) to investigate the illnesses. The two passengers were diagnosed after traveling on the vessel late last year.
What Is Legionnaires’ Disease?
Legionnaires’ disease is a serious type of pneumonia caused by Legionella bacteria. According to the cruise line’s letter, people become ill when they inhale tiny water droplets that contain the bacteria.
Unlike many respiratory infections, Legionnaires’ disease does not spread from person to person. Instead, the bacteria can grow in water systems and occasionally spread through mist or vapor from fixtures such as hot tubs, showers, whirlpool bathtubs, decorative fountains and misters.
The CDC notes that symptoms usually appear between two and 14 days after exposure, though in some cases they may take longer. The illness often resembles pneumonia and can include cough, fever, headache, muscle aches and shortness of breath. Some patients may also experience confusion, diarrhea or nausea.
While most people exposed to Legionella bacteria do not become sick, certain groups face a higher risk. Adults over 50, current or former smokers and individuals with underlying health conditions are considered more vulnerable to severe infection.
Investigation Underway, Tests So Far Negative
In its communication to passengers, Norwegian Cruise Line said it has launched onboard testing for Legionella bacteria. The testing includes water systems and fixtures such as hot tubs, showers and faucets.
“So far, all test results have been negative,” the company stated. It added that it remains unclear whether the two passengers were exposed through the ship’s water system or from another source unrelated to the cruise.
The cruise line urged anyone currently sailing or who recently disembarked from the Norwegian Encore to monitor their health. Guests who develop symptoms within 14 days of the end of their cruise have been advised to contact the ship’s medical staff and seek immediate medical attention.
CDC Monitoring the Situation
In a statement issued February 13, a spokesperson for the CDC confirmed to PEOPLE that the agency is aware of the cases associated with the Norwegian Cruise Line vessel and is supporting the investigation.
The development follows a separate CDC report released in October 2024 that examined 12 cases of Legionnaires’ disease identified among travelers on two cruise ships between November 2022 and June 2024. In that earlier investigation, private balcony hot tubs were identified as the likely source of exposure in two outbreaks.
The CDC noted that private hot tubs are often subject to less stringent operating requirements than public ones. The agency also warned that certain features — including outdoor placement, water retention between uses and systems involving recirculation, filtration or heating — can increase the risk of Legionella growth and transmission.
For now, health officials and the cruise line continue to investigate the source of the recent cases, as passengers are reminded to remain alert to symptoms and seek prompt care if they feel unwell.
Frida Baby Thermometer Under Scrutiny After Brand Sexualizes Its Packaging
Credits: Canva (Representational)
Frida Baby thermometer is facing severe backlash after parents criticized the company's marketing language on its packaging. The parents claimed that the company cracked sexual jokes on using baby thermometers and that it crossed a line for a product that was designed for infants.
The controversy gained more traction this week after a social media user posted about it on X with photos stating that the marketing relies on sexual jokes.
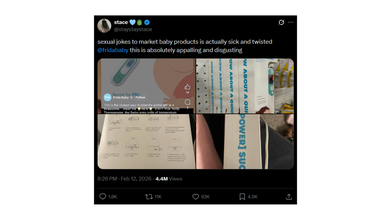
An X user @staystaystace wrote: "sexual jokes to market baby products is actually sick and twisted @fridababy this is absolutely appalling and disgusting".
Frida Baby Thermometer: What Was The Problem With Marketing

The photos include a screenshot of the thermometer packaging on which the graphics are pointed out to be problematic and inappropriate by the parents. Furthermore, the caption on the official account of Frida Baby social media reads: 'This is the closest your husband's gonna get to a threesome...'
In another photo of Frida Baby 3-in-1 Eat, Forehead and Touchless Thermometer, the marketing phrase reads: 'How About A Quickie?'
Another photo on steps for using the humidifier, the caption on the packaging reads: 'I Get Turned On Easily'. While a fourth photo reads: 'I'm A [Powerful] Sucker'.
Parents claim that the brand has used sexual market phrases found similarly on self care toys and massagers for products which are made for infants.
Read: Six-year-old Child Dies Of Medical Negligence During MRI At Greater Noida Imaging Centre
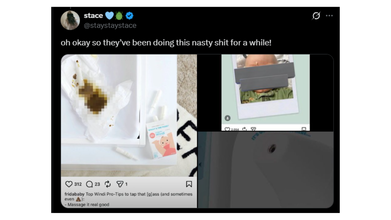
Amid this, older content from 2020, including deleted social media posts resurfaced featuring a baby with visible nose discharge and the caption read: 'What Happens When You Pull Out Too Early #nosefrida #dontmove'.
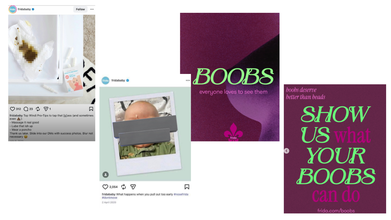
More recently, Frida Baby again drew attention for an Instagram post that centered on breastfeeding. The carousel post includes slides with statements like: 'Boobs, everyone loves to see them', followed by commentary on how breasts are widely accepted in pop culture, but criticized in the context of feeding a child. The final slide reads: 'Show Us What Your Boobs Can Do', which directs readers to company's website. A user on the Instagram post commented: "Hey so why do you sexualize your products??"
Another photo that resurfaced from 2021 reads: 'Top Windi Pro-Tips to tap that [g]ass (and sometimes even [poop emoji]):
- - Massage it real good
- - Lube that ish up
- - Wear a poncho
However, there were some parents who also defended the brand's tone and argued that humor is clearly aimed at adults who are navigated through the realities of parenting.
Frida Baby Thermometer: How Did The Brand React?
In an emailed statement, as reported by Complex, the spokesperson for the brand said:
From the very beginning, Frida has used humor to talk about the real, raw, and messy parts of parenting that too often go unspoken. We do this because parenting can be isolating and overwhelming, and sometimes a moment of levity is what makes a hard experience feel human, shared, and survivable.
Our products are designed for babies, but our voice has always been written for the adults caring for them. Our intention has consistently been to make awkward and difficult experiences feel lighter, more honest, and less isolating for parents.
That said, humor is personal. What’s funny to one parent can feel like too much to another. We’re never trying to offend, push boundaries for shock value, or make anyone uncomfortable. Importantly, our tone is never separate from our product. The humor we use is always grounded in a specific feature, benefit, or innovation — a reflection of the real problem we are solving for families.
Frida was built to support families through some of the most vulnerable and transformative chapters of their lives. We stand firmly behind that mission. We will continue to show up with honesty, empathy, and courage.
With each decision we make, we will continue to evaluate how we express our voice so that our commitment to families is unmistakable and our tone always meets the moment.
Six-year-old Child Dies Of Medical Negligence During MRI At Greater Noida Imaging Centre
Credits: Canva
A six-year-old boy died after his health worsened during an MRI scan at a private Greater Noida diagnostic centre. His family alleged medical negligence and claimed that he was administered a wrong or heavy dose injection.
As per the boy's father, Prashant Kasana, his son was taken to the centre for some test and was given an injection before the MRI procedure. The family said that during the MRI scan, the child was administered a heavy dose. Due to which his condition worsened and he also lost consciousness.
The family also said that when they asked for information about the child's condition and his medical report, they were not given any satisfactory answers. They also claimed that the doctors gave another dose to the child. The child's condition did not improve and the family had to rush the child to another nearby private hospitals. This is where the doctors declared him dead.
Six-year-old Child Dies of Medical Negligence: What Happened?
After this incident, family members accused the staff of the KB Healthcare Centre, where the child was first taken for an MRI scan. Villagers and workers of the Bharatiya Kisan Union also reached the spot and staged a protest.
The state spokesperson of the Bharatiya Kisan Union, Pawan Khatana, stated that the child was brought to the centre at 10.30am, and was in normal condition. As per the spokesperson, doctors did not disclose the quantity of the dose administered to the child.
As per Khatana, even after half an hour, the child did not gain consciousness and when the doctors checked him again, he was unresponsive and cold. The family took to another private hospital where he was declared dead. Khatana also alleged that there are many such unauthorized screening and imaging centres operating in Greater Noida and demanded a thorough probe.
Six-year-old Child Dies Of Medical Negligence: What Are The Authorities Saying?
Police on reaching the spot received the information, while protesters demanded for a fair investigation and strict action against those responsible. The Station House Officer of Neta 2 police station said the child was a resident of Reelkha in Dankaur. He was brought to a private pathology lab in Sector P3 for an MRI scan. As per the officer, the doctor administered the child with anesthesia for an MRI. After this, the child's health started to deteriorate.
Police has sent the body for post-mortem after completing the necessary legal formalities.
Six-year-old Child Dies of Medical Negligence: How Much Anesthesia Is Safe For A Child During MRI?
As per the National Institutes of Health (NIH), US and a study by the Yeungnam University Journal of Medicine (YUJM), drugs for deep sedation or general anesthesia for pediatric MRI are:
(I) Chloral hydrate: a sedative hyptonic agent with no analgesic properties
The study notes that the recommended dose of chloral hydrate is 50 to 100 mg/kg, or up to a maximum of 2g. The success rate of chloral hydrate sedation for pediatric MRI varies from 78% to 100%.
The United Kingdom (UK) National Institute for Clinical Excellence (NICE) also recommends the use of oral chloral hydrate with a wide margin of safety in children under 15 kg.
The study also notes that children "may be encouraged to take at least clear fluids 2 hours before the procedure for successful sedation without breaking institutional fasting protocols for chloral hydrate sedation".
(II) Pentobarbital: a medium duration barbiturate that provides potent sedation with no analgesic property.
As per the study, this can be administered via an oral or intravenous or IV route. The oral dose is administered between 4 to 8mg per kg and IV dose of 2 to 3 mg per kg.
(III) Midazolam: it is a short-acting water soluble benzodiazepine that has anxiolytic, sedative, amanestiec, and muscle relaxant properties.
It is administered through various routes, but IV is preferred. When administered through IV, it is given at the dose of 0.1mg per kg.
Anesthetic agents include propofol and sevoflurane.
Note: This article is not a substitute for medical consultation or prescription. The information is based on reports and research articles available online for public.
© 2024 Bennett, Coleman & Company Limited

