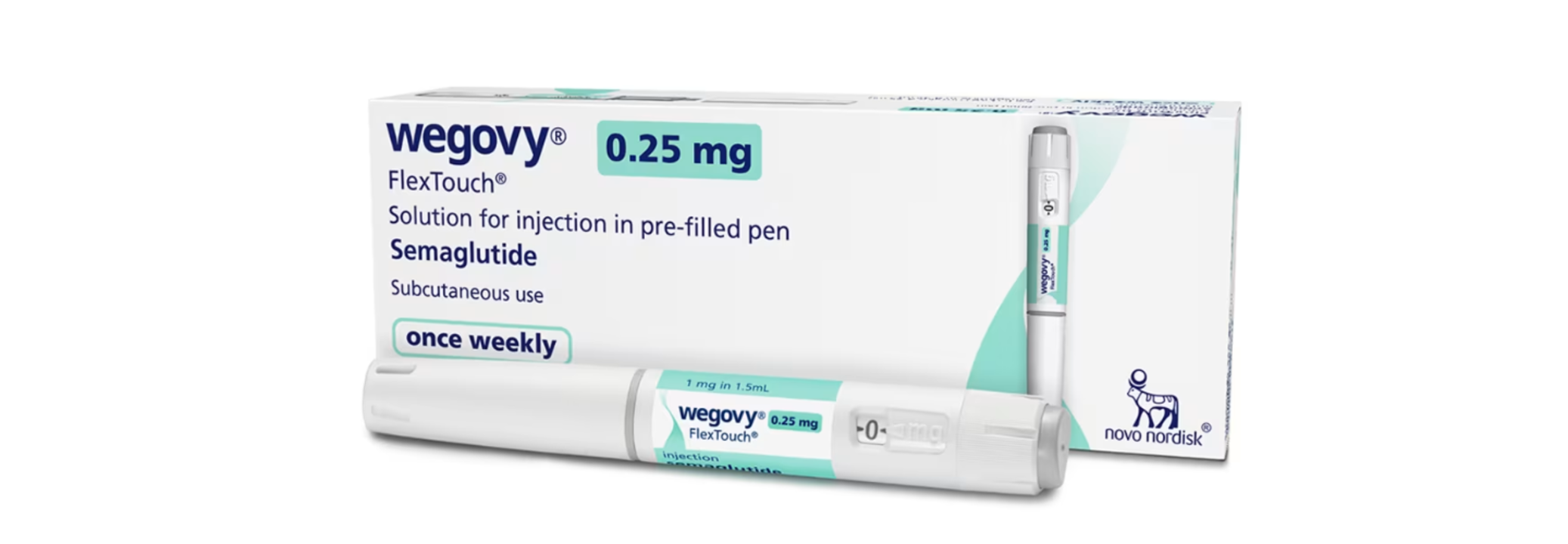
Novo Nordisk Launches Bestseller Weight Loss Drug Wegovy In India
Credits: Novo Nordisk
SummaryNovo Nordisk launches its FDA-approved weight-loss drug Wegovy in India, rivaling Eli Lilly’s Mounjaro, as obesity and diabetes rise. Clinical trials show significant weight loss with weekly injections.
End of Article
