- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Whooping Cough Outbreak In USA: Warning Signs And Prevention Tips You Need To Know

Whooping cough cases in USA
Centers for Disease Control and Prevention The Centers for Disease Control and Prevention, CDC, recently reported that whooping cough, or pertussis, cases around the United States have surged this year and thus returned to pre-pandemic levels. According to the CDC, as of October 5, 2024, there were 17,579 reported cases of whooping cough, a steep increase compared to the 3,962 reported by that date last year.
This spiking indicates that it has surged by more than 13,600 cases to put current figures back in line with those recorded before the COVID-19 pandemic.
The bacterium Bordetella pertussis causes whooping cough, posing the highest risk to infants less than one year old, whose immature immune systems make them very vulnerable to serious illness or death. These cases continue to comprise the majority of reported cases of pertussis according to a CDC report.
Symptoms to Watch for
Whooping cough begins with symptoms that may be mistaken for the common cold. Symptoms most often begin within 5 to 10 days from when a person first starts to show signs of being infected, but this can be as long as three weeks in some cases. Early warning signs include:
- Runny or stuffy nose
- Mild fever
- Slight, intermittent cough
For a newborn, some symptoms are severe, including labored or dangerous pauses in breathing (apnea). Babies may turn blue with this disease.
In more serious cases of the disease, these give way to other more serious symptoms. These are as follows:
- Rapids, violent, and uncontrollable coughing fits
- High-pitched "whoop" sounds during inhalation
- Vomiting would develop, sometimes at the time of or following coughing spasms
- Fracture the ribs
Whooping Cough Prevention: Vaccination and Hygiene
The CDC suggests that vaccination is the best form of prevention of whooping cough. There are two vaccines: DTaP vaccine for children and Tdap for adolescents and adults. However, even vaccinated patients can get infected because the immunity from the vaccine gradually decreases with time. Booster shots are thus essential in keeping one updated.
In addition to vaccination, hygiene practices are a significant step in not transmitting the whooping cough. One must frequently wash hands, especially after the coughing and sneezing, and always cough and sneeze with the mouth and nose covered to avoid airborne transmission.
Protect Yourself and Your Family: Tips to Stay Safe
Seeking medical attention immediately and following the healthcare provider's instructions in case you or your family member develops symptoms of whooping cough. The disease is frequently mild and will clear itself, but early initiation with antibiotics may reduce the severity and limit its spread to others.
For the families having infants or weaker members in the household, precautions should be increased, especially since all eligible members of the family must have their vaccinations up to date. As an infant is one of the most vulnerable ones, taking care to keep the home environment clean, smoke-free, dust-free, and fume-free will make him feel much better and keep away the coughing fits.
Cool mist humidification can help the patient by loosening mucus and soothing the cough. Small, frequent meals can prevent some of the vomiting with coughing fits. Fluids must be copious, including water, as well as other fluids such as fruit juices.
Finally, early diagnosis and proper treatment, in addition to prevention and prevention measures, such as vaccination, hygiene, among others, are the best tools to control whooping cough and protect your family from this dangerous disease.
First Symptom Of Alzheimer’s Is Triggered By This Signal

Credits: iStock
A fading sense of smell may do more than hint at aging—it could be one of the earliest warning signs of Alzheimer’s disease. New research from DZNE and Ludwig-Maximilians-Universität München reveals that immune cells in the brain mistakenly target and destroy nerve fibers critical for odor perception, offering fresh clues into how the disease begins and how it might be diagnosed sooner.
Patients and families may notice that scents seem muted or distorted, sometimes years before other symptoms. Until now, the exact cause behind this early warning sign remained elusive.
A new study led by researchers at the German Center for Neurodegenerative Diseases (DZNE) and Ludwig-Maximilians-Universität München (LMU) sheds fresh light on the mystery. The findings, published in Nature Communications, suggest that the brain’s own immune system mistakenly destroys nerve fibers vital for processing odors. This breakthrough could open the door to earlier diagnosis—and possibly earlier treatment—of Alzheimer’s.
How the Brain Processes Smell?
Smell perception begins in the olfactory bulb, a small but complex structure in the forebrain that receives input from sensory receptors in the nose. But the olfactory bulb doesn’t work alone. It relies on nerve fibers extending from the locus coeruleus, a brainstem region that helps regulate attention, blood flow, and sensory processing.
The new study shows that in early Alzheimer’s disease, this communication line is disrupted. Microglia—immune cells that normally act as the brain’s cleanup crew—start dismantling the nerve fibers linking the locus coeruleus and olfactory bulb. This immune-driven attack deprives the brain of crucial odor-processing pathways, leading to smell loss.
“Our study suggests that in early Alzheimer’s disease, changes occur in the nerve fibers linking the locus coeruleus to the olfactory bulb. These alterations signal to the microglia that affected fibers are defective or superfluous. Consequently, the microglia break them down,” explained Dr. Lars Paeger of DZNE and LMU.
What Is The “Eat-Me” Signal That Triggers Alzheimer’s?
At the heart of this immune misfire lies an unusual molecular change. Researchers observed that phosphatidylserine, a fatty acid normally tucked inside the protective membrane of neurons, shifts to the cell’s outer surface in affected nerve fibers.
When this happens, microglia interpret it as an “eat-me” signal. Under normal conditions, this signal supports a healthy process called synaptic pruning, where unnecessary or damaged connections are cleared away. But in Alzheimer’s, this mechanism seems to go awry.
Paeger explained that hyperactive neurons—cells firing abnormally due to early disease changes—appear to trigger this membrane shift. Once flagged as dysfunctional, these otherwise critical smell-related fibers are targeted and destroyed.
PET imaging scans in living patients, which confirmed damage to smell-related nerve circuits in people with Alzheimer’s or mild cognitive impairment.
“Smell issues in Alzheimer’s disease and damage to the associated nerves have been discussed for some time. However, the causes were unclear until now. Our findings point to an immunological mechanism as cause for such dysfunctions – and, in particular, that such events already arise in the early stages of Alzheimer’s disease,” said Prof. Joachim Herms, senior researcher on the study.
Why Smell Loss Is Important in Diagnosis of Alzheimer’s?
More than 6 million Americans currently live with Alzheimer’s disease, a number projected to rise sharply as populations age. Yet diagnosis often comes late, when memory loss and cognitive decline are already advanced.
Smell loss offers an earlier red flag. If the biological mechanisms behind it can be mapped and measured, doctors could potentially screen for Alzheimer’s years before symptoms interfere with daily life. This could be especially critical as new treatments, such as amyloid-beta antibodies, are designed to work best in the earliest phases of disease progression.
“Our findings could pave the way for the early identification of patients at risk of developing Alzheimer’s, enabling them to undergo comprehensive testing to confirm the diagnosis before cognitive problems arise,” Herms noted. “This would allow earlier intervention with amyloid-beta antibodies, increasing the probability of a positive response.”
The study also highlights the often-overlooked locus coeruleus, a small cluster of neurons deep in the brainstem. Beyond smell, this structure regulates blood flow, sleep-wake cycles, and stress responses through widespread connections.
Damage to the locus coeruleus is one of the earliest detectable signs of Alzheimer’s, even before amyloid plaques and tau tangles spread widely. The new findings underscore that its breakdown may not only affect memory and attention but also disrupt sensory pathways, making smell loss one of the first noticeable symptoms.
Can Sense of Smell Be Preserved?
While the research answers critical questions, it also raises new ones. If immune cells misidentify smell-related fibers as expendable, can this process be slowed or stopped? Could therapies aimed at stabilizing neuron membranes prevent the fatal “eat-me” signal from being displayed in the first place?
Future studies will likely explore whether drugs that regulate microglial activity—or protect the integrity of phosphatidylserine positioning—could preserve nerve connections in early Alzheimer’s.
Meanwhile, incorporating smell testing into routine checkups for older adults may become a more practical step. Inexpensive scratch-and-sniff exams already exist and could serve as a noninvasive screening tool to identify people who may need further evaluation.
For families watching loved ones struggle with subtle changes—whether misplaced keys, unusual forgetfulness, or an inability to smell morning coffee—the findings offer clarity. Smell loss is not just an odd, isolated symptom but a biological signal that Alzheimer’s is affecting the brain long before dementia sets in.
Recognizing this signal early could give patients and clinicians a window of opportunity: time to prepare, time to plan, and perhaps in the future, time to intervene with treatments that slow or alter the course of disease.
Alzheimer’s remains one of the greatest public health challenges of our time. While there is still no cure, understanding its earliest signals brings us closer to meaningful change. By uncovering how immune cells mistakenly dismantle smell pathways, this study not only solves a long-standing puzzle but also lays the foundation for earlier, more effective care strategies.
The sense of smell may be more than just a window to the world—it may be a window into the earliest changes of Alzheimer’s disease.
Tiny 3D-Printed Spinal Cords Could Reverse Paralysis, How Did Scientists Make It Work?
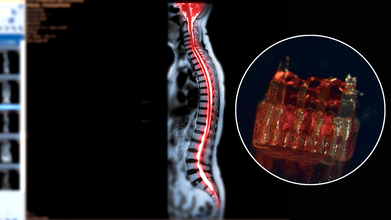
Credits: Canva/McAlpine Research Group, University of Minnesota
Spinal cord injuries have long posed one of the most stubborn challenges in medicine. Affecting more than 300,000 people in the United States alone, these injuries often lead to permanent paralysis because damaged nerve fibers fail to regenerate across the site of trauma. Traditional therapies focus largely on rehabilitation and symptom management rather than reversing the underlying injury. Now, a groundbreaking study from the University of Minnesota Twin Cities suggests that a combination of 3D printing, stem cells, and lab-grown tissues could change that narrative. Researchers have engineered tiny scaffolds that guide stem cells to form nerve fibers capable of bridging severed spinal cords. In rat models, this approach restored nerve connections and movement—offering a tantalizing glimpse into the future of paralysis treatment.
At the heart of this innovation are organoid scaffolds—microscopic 3D-printed structures designed to direct stem cell growth. These scaffolds contain a network of tiny channels that can be seeded with spinal neural progenitor cells (sNPCs). Originating from human adult stem cells, sNPCs have the potential to differentiate into the various types of neurons needed for spinal cord repair. The scaffold essentially provides a framework for these cells, ensuring they grow along the correct pathways to reconnect disrupted nerve circuits.
Guebum Han, a former postdoctoral researcher in mechanical engineering at the University of Minnesota and the study’s first author, explains, “We use the 3D printed channels of the scaffold to direct the growth of the stem cells, which ensures the new nerve fibers grow in the desired way. This method creates a relay system that, when placed in the spinal cord, bypasses the damaged area.”
To test their approach, the researchers transplanted the scaffolds into rats with completely severed spinal cords. Over time, the stem cells differentiated into mature neurons and extended their nerve fibers in both directions—toward the head (rostral) and toward the tail (caudal)—forming new connections with the host’s existing spinal circuitry.
The results were remarkable. Rats that received the organoid scaffolds showed significant functional recovery compared to controls, regaining movements that were previously impossible. The new neurons integrated seamlessly into the host tissue, demonstrating that lab-grown spinal tissue could not only survive transplantation but also restore communication across previously severed areas.
Can Lab-Grown Organs Help Patients?
While the research is still in its early stages, the potential implications for human medicine are profound. Spinal cord injuries have been notoriously resistant to treatment because adult nerve cells rarely regrow once damaged. This study provides proof-of-concept that targeted, scaffold-guided stem cell growth can rebuild the neural network necessary for motor function.
Ann Parr, professor of neurosurgery at the University of Minnesota, emphasizes the significance: “Regenerative medicine has brought about a new era in spinal cord injury research. Our laboratory is excited to explore the future potential of our ‘mini spinal cords’ for clinical translation.” The team hopes to refine the technique, scale up scaffold production, and move toward clinical trials that could one day benefit people living with paralysis.
Despite the promising results, several hurdles remain before this technology can be applied to humans. Scaling the tiny lab-grown spinal cords to the size necessary for human injuries will require sophisticated bioengineering solutions. Immune rejection and integration into a complex, pre-existing nervous system present additional challenges. Moreover, safety and efficacy will need to be rigorously tested in larger animal models before human trials can proceed.
The ethical considerations of stem cell use and genetic manipulation also require careful navigation. While adult stem cells used in this study bypass some of the ethical debates associated with embryonic stem cells, clinical applications must still adhere to stringent regulatory standards.
The merging of 3D printing, stem cell science, and lab-grown tissue engineering represents a paradigm shift in regenerative medicine. The concept of “mini spinal cords” could open the door to therapies not only for spinal cord injuries but potentially for other neurodegenerative diseases that involve nerve degeneration, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis.
Moreover, these technologies exemplify the broader trend of personalized medicine. By tailoring organoid scaffolds to individual patients, it may become possible to repair nervous system injuries with unprecedented precision. This could drastically improve outcomes, reduce rehabilitation times, and enhance quality of life for patients who currently have few options.
The University of Minnesota study is an early but significant step toward reversing paralysis. By combining 3D-printed scaffolds, stem cell biology, and lab-grown spinal tissue, researchers have demonstrated that damaged neural pathways can be rebuilt and functional recovery is achievable—at least in animal models.
While human applications are still a way off, the research provides a blueprint for the future of spinal cord repair and regenerative neuroscience. For the millions affected by spinal cord injuries, these tiny lab-grown spinal cords could one day offer more than hope—they could offer a pathway to regained movement and independence.
Hot Mic Moment: What Putin, Xi Said About 'Living Indefinitely'; Can Humans Really Live For 150-Years?

Credits: Reuters/Shutterstock/AI
At a military parade in Beijing, an open-mic moment between Chinese President Xi Jinping and Russian President Vladimir Putin revealed an unusual exchange. The leaders were overheard discussing organ transplants and the possibility of dramatically extending human life. Putin even raised the prospect of “eternal life” through biotechnology, according to translated remarks aired on Chinese state TV. The conversation, captured as Xi, Putin, and North Korean leader Kim Jong Un walked through Tiananmen Square, has fueled fresh debate on the limits of science and longevity. Both Xi and Putin, in power for 13 and 25 years respectively, have shown no signs of stepping down.
The pageantry was meant to project strength, but a stray hot mic gave the world something unexpected: an intimate glimpse into how two of the most powerful men on Earth think about the human lifespan.
Putin’s interpreter was caught saying, “Biotechnology is continuously developing. Human organs can be continuously transplanted. The longer you live, the younger you become, and even achieve immortality.” Xi responded, almost casually: “Some predict that in this century humans may live to 150 years old.”
The microphones were quickly faded out, and the livestream camera cut away. But the brief exchange rippled far beyond the parade. Could 150 really be the ceiling for human life—or even a realistic milestone?
What is The Hot Mic Moment?
Both Xi and Putin later confirmed the conversation, with Putin remarking to Russian media that new medical advances, including organ replacement, could extend “active life” significantly. For leaders who have each been in power for more than a decade—and show no signs of stepping aside—the notion of extending life carries not just personal but political undertones.
This wasn’t idle speculation in a vacuum. Russia, China, and the United States are all investing heavily in biotechnology, regenerative medicine, and artificial intelligence as tools not only of economic growth but also of national prestige. Against that backdrop, Xi’s mention of 150 years sounded less like science fiction and more like a pointed acknowledgment of what researchers are seriously debating.
How Long Do Humans Live Now?
At present, global life expectancy hovers around 73 years, with wealthier countries averaging in the low 80s. The record for the oldest documented human belongs to Jeanne Calment, a Frenchwoman who lived to 122 before passing in 1997.
But it’s important to separate two concepts: average life expectancy (how long most people live, heavily influenced by disease, healthcare access, and environment) and maximum lifespan (the theoretical upper limit of human survival under ideal conditions). Average life expectancy has steadily climbed over the past century thanks to vaccines, antibiotics, improved sanitation, and better maternal care. Maximum lifespan, by contrast, has barely budged.
That’s why Xi’s remark matters. He wasn’t talking about incremental gains—he was floating the possibility of breaking through a biological barrier.
Can Humans Live As Long As 150 Years?
A team of scientists from Singapore, Russia, and the United States recently modeled human resilience using blood samples from more than 70,000 people aged up to 85. They tracked fluctuations in white and red blood cells to create a measure called the Dynamic Organism State Indicator (Dosi), which captures the body’s ability to recover from stress and illness.
Their conclusion was striking: resilience collapses completely around age 150, setting an upper bound for human lifespan. This wasn’t a forecast of what people will achieve anytime soon—it was a theoretical ceiling based on current biology.
Put simply, the study suggests that even if you dodge heart disease, cancer, infections, and accidents, your body will eventually lose its ability to recover. That’s the point at which life becomes unsustainable.
Is Living Till 150 Years Plausible Or Out of Reach?
From a biological standpoint, the 150-year limit makes sense. Organs age, stem cells lose regenerative capacity, and the body accumulates damage at the cellular level. At present, most human organs seem capable of functioning for 110 to 120 years under ideal conditions. Beyond that, decline accelerates.
Yet science is moving fast. Regenerative medicine, organ transplantation, and lab-grown tissues could shift the baseline. Already, researchers have extended the lives of worms tenfold and mice by 30–40%. Humans, with more complex biology, are harder to push, but the trajectory suggests improvement is possible.
Still, adding 25–30 years of healthy life is far more realistic in the near future than reaching 150. As one researcher quipped, “We might not see immortality, but 110 could become the new 90.”
Evidence from real-world populations supports the idea that genetics and lifestyle can push lifespans well beyond the norm. The “Blue Zones”—regions like Okinawa in Japan and Sardinia in Italy—produce unusually high numbers of centenarians. Their secrets aren’t futuristic: plant-heavy diets, daily movement, strong community ties, and low chronic stress.
Even so, these populations rarely see people surpassing 110. Jeanne Calment remains an outlier, not a model. Which raises the question: are biology and environment already giving us the maximum return, or is medicine the only path to break through?
What Role Does Biotechnology Play?
This is where Putin’s remark about organ transplants comes in. Medicine has already normalized replacing failing hearts, kidneys, and joints. Stem cell therapies are being tested to repair damaged tissues. Artificial intelligence is accelerating drug discovery. CRISPR and gene editing open the possibility of correcting mutations that drive aging and disease.
Theoretically, if each organ could be replaced or rejuvenated before it fails, the body could remain younger for longer. The challenge is that aging is systemic. Replacing a heart doesn’t stop immune decline, nor does repairing a kidney fix memory loss. The body doesn’t age in silos; it ages all at once.
How Can Political Leaders Shape The Theory of Longevity?
It’s no accident that authoritarian leaders are voicing interest in radical life extension. Both Xi and Putin oversee nations that pour resources into biotechnologies, often with fewer ethical guardrails than in the West. Extending human lifespan is not just a health goal—it’s a geopolitical lever, a way to showcase scientific dominance.
At the same time, public health experts caution against letting these conversations distract from pressing needs. In countries where average life expectancy still lags below 70, access to vaccines, clean water, and chronic disease care would do far more for human survival than speculative anti-aging research.
For now, the 150-year limit is a provocative talking point, not a practical horizon. But Xi and Putin’s hot mic exchange underscores something deeper: longevity science has moved from the fringes to the geopolitical stage.
If history is any guide, the biggest gains won’t come from science fiction-style immortality but from steady, incremental progress—cutting smoking rates, controlling hypertension, managing diabetes, and ensuring equitable access to healthcare. These measures, not transplants or futuristic drugs, are what extend average life expectancy year after year.
The mic may have been unguarded, but the conversation it captured was anything but trivial. Leaders of two nuclear powers spoke openly about the possibility of living indefinitely. While the science points to 150 years as a theoretical ceiling, the more pressing challenge for humanity is not how long we can live, but how well.
As researchers continue probing the biology of aging, the lesson from Blue Zones and centenarians alike still rings true, a balanced diet, physical activity, meaningful social ties, and preventive healthcare remain the closest things we have to longevity secrets. The road to 150 may or may not be real, but the path to 100—healthy, independent, and vibrant—is already within reach.
© 2024 Bennett, Coleman & Company Limited

