- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
‘Workout Queen- The Epitome Of Health’ Dies At 28, After Suffering Caffeine Triggered Heart Attack And Fatal Brain Damage

Image Credit: Canva
Katie Donnell, a 28-year-old teacher from Florida, was the epitome of health- followed a rigorous fitness routine, ate only organic foods, and had no diagnosed medical conditions. But in August 2021, her life was suddenly cut short by a heart attack. Her grieving mother, Lori Barranon, now blames one daily habit which is rather an unsuspected factor that may have contributed to her daughter's premature death—too much caffeine.".
In spite of her devotion to a fit lifestyle, Donnell drank several energy drinks per day, along with coffee and pre-workout caffeine supplements. Although medical doctors did not link her death directly to caffeine, Barranon is certain that her daughter's dependency on these drinks had deadly results.
Donnell was hanging out with friends when she simply collapsed, her eyes rolling back in her head as she lost consciousness. At first, people assumed she was having a stroke. Emergency workers came in and tried to bring her back around, but it was too late. She had not received oxygen for too long a period of time and suffered from massive brain damage. After three hours of medical treatment, she never did come around.
Physicians put Donnell into a medically induced coma in hopes of stabilizing her situation, but she had worsening seizures during the ensuing days. Following ten days of life support, her family was forced to make the sad decision to release her.
Risks of Over-Caffeination
Though caffeine is common in increasing alertness and energizing, it has fatal results when used in excess. The medical fraternity advises that excessive caffeine can elevate blood pressure, boost heart rate, and lead to cardiovascular problems. Caffeine's ability to acutely elevate blood pressure can strain the cardiovascular system, enhancing the possibility of heart arrhythmias and cardiac arrest, warns the National Library of Medicine.
The Mayo Clinic says that a daily intake of up to 400 milligrams of caffeine is generally safe for adults. This would be the equivalent of about four small cups of coffee, five or six shots of espresso, or two to four large store-bought coffee drinks. Energy drinks, though, contain between 100 and 300 milligrams of caffeine per can, so having multiple energy drinks a day in addition to coffee or supplements is an easy way to exceed the safe amount.
In Donnell's case, the amount of caffeine that she consumed daily was shocking. Barranon subsequently found that her daughter would buy four-packs of energy beverages twice or three times a week, aside from consuming massive quantities of coffee. Her vehicle had several packs of empty energy drink cans, indicating a reliance on these products.
Pattern of Caffeine-Related Health Incidents
Donnell's case is not the only one. In 2018, 21-year-old Australian musician Lachlan Foote passed away due to caffeine overdose after he added caffeine powder to his protein shake. Recently, in 2023, a 20-year-old Jazmin Garza was put on life support after drinking only a few sips of an energy drink before exercising, according to GoFundMe page. These events underscore the possible risks of excessive consumption, particularly among youth who are not yet fully cognizant of the risks.
While moderate use of caffeine can be beneficial in terms of enhanced concentration and enhanced physical performance, excessive use can cause severe health complications. Side effects include heightened heart rate, elevated blood pressure, anxiety, and, in extreme instances, heart attacks or sudden cardiac arrest.
Barranon is now on a crusade to inform others of the secret dangers of energy drinks and caffeine supplements. She implores parents to keep their children's intake in check, cautioning that too much caffeine can have catastrophic effects.
If you don't keep your children away from this stuff, you might be in my position where your life is destroyed," she said. "It's so dangerous and lethal. This is affecting my entire family.
Barranon now goes out of her way to warn others whenever she notices a person with an energy drink in their hand. "I beg people to advise their children and observe what they're doing. I was watching Katie, but I had no idea of the extent of it."
What Is Caffeine Intoxication?
Caffeine intoxication, or caffeine overdose or toxicity, is the condition that results from excessive caffeine intake and leads to adverse effects on the body. Although caffeine is commonly ingested in the form of coffee, tea, soda, and energy drinks, excessive intake has the potential to result in serious health hazards.
The majority of Americans ingest caffeine every day, with moderate use having positive effects such as heightened alertness and energy. But when the amount of caffeine is beyond what the human body can metabolize, it can lead to symptoms that vary from mild pain to severe medical issues.
How Much Caffeine Is Too Much?
As stated by the U.S. Food and Drug Administration (FDA), adults can safely consume 400 milligrams of caffeine daily—about the equivalent of two to three 12-ounce cups of coffee. Caffeine sensitivity, however, differs among individuals, and medical conditions and medications can enhance sensitivity.
For kids, the dangers are even greater. Although there is no official safe amount for children, experts warn strongly against the use of caffeine in children younger than 2 years and urge children and teens to steer clear of energy drinks entirely.
Regulating Energy Drink Consumption: What Can Be Done?
The widespread distribution of high-caffeine energy beverages has created increasing concerns among health practitioners. In many nations, there are controls on the sale of energy drinks to minors, but caffeine-related health issues are extensively under-discussed.
Public health experts point out the importance of clearer labelling on energy drinks, outlining their levels of caffeine and associated risks. Some are calling for stricter regulations, such as age limits and warnings regarding the perils of overuse of caffeine.
For those who rely on caffeine to make it through the day, professionals advise replacing it with safer sources, like herbal teas or natural energy sources such as balanced nutrition and adequate hydration. Fitness buffs, in particular, need to exercise caution when using pre-workout supplements that have excessive amounts of caffeine and other stimulants.
Katie Donnell's sad tale is a harsh reminder that even seemingly innocuous habits can have life-changing repercussions. Although caffeine is a ubiquitous and socially tolerated stimulant, overconsumption—particularly when combined with intense exercise—can place extreme stress on the heart.
While energy drinks are more popular than ever, it is essential that everyone, parents in particular, as well as policymakers, take a closer examination at their possible effects on health. Through increased awareness and education for responsible use, such tragedies as Donnell's can be avoided in the future.
For the time being, Barranon continues to tell her daughter's story hoping that it will be a warning to others. "I don't want anyone else to experience this suffering. If I can save even one life, then Katie's story won't be for nothing."
Nasal Spray That 'Works In Hours' May Replace Pills For Depression Treatment

Credits: Canva
A breakthrough study finds esketamine nasal spray may deliver meaningful improvement for treatment-resistant depression within hours. For decades, patients struggling with major depressive disorder (MDD) have had to wait weeks sometimes months for antidepressant medications to show effects. For roughly one in three people, even after trying multiple drugs, relief never comes. This group, known as treatment-resistant depression (TRD), faces limited and often frustrating options.
Now, a new clinical trial suggests there may be another path, esketamine nasal spray. Researchers report that when used as a standalone treatment, esketamine provided rapid and sustained relief for adults with TRD. Unlike traditional antidepressants that take weeks to work, improvements appeared within 24 hours and lasted throughout the study’s four-week duration. The findings were published in JAMA Psychiatry.
Ketamine, a compound first approved in the 1970s as an anesthetic, has drawn increasing attention for its antidepressant effects at lower doses. Esketamine, a chemically refined version, works on similar pathways but is more targeted.
In 2019, the U.S. Food and Drug Administration approved SPRAVATO, an esketamine nasal spray, for use alongside oral antidepressants in TRD. Until now, it was unclear whether the spray could stand on its own. This new phase 4 trial offers the strongest evidence yet that esketamine may be effective without an added oral drug.
The study enrolled 378 adults across 51 U.S. outpatient centers between 2020 and 2024. To qualify, participants had to show a history of poor response—defined as less than 25% improvement—to at least two antidepressants during their current depressive episode.
Key features of the trial included:
Design: randomized, double-blind, placebo-controlled
Groups: fixed doses of either 56 mg or 84 mg esketamine, or a placebo spray
Treatment schedule: twice-weekly dosing for four weeks
Primary measure: changes in Montgomery-Åsberg Depression Rating Scale (MADRS) scores, which capture depression severity
Most participants were women (61%), and the average age was 45. All entered the study with moderate-to-severe depression.
How Esketamine Nasal Spray Works?
Within a single day of treatment, both esketamine groups reported noticeable improvements compared to placebo. By day 28, those on 56 mg showed a 5.1-point greater reduction in symptom scores versus placebo.
The 84 mg group had an even stronger effect, with a 6.8-point advantage. For perspective, traditional antidepressants often take six to eight weeks to yield measurable changes. Esketamine’s speed is one of the factors making it so promising for TRD patients, many of whom live with intense, persistent distress.
Patients who continued into the optional 12-week open-label phase—where all participants received esketamine—maintained or further improved their scores. This suggests the drug’s benefits may extend beyond the initial four weeks.
The “number needed to treat,” a measure of clinical significance, was around 6–7 for symptom response. This means that for every six or seven patients treated, at least one more experienced a meaningful reduction in depression compared to placebo. For a psychiatric intervention, that is a robust effect size.
Are There Any Side Effects of the Nasal Spray?
Like other ketamine-based therapies, esketamine is not without side effects. In this trial, the most common were nausea, dizziness, headache, and dissociation, a temporary feeling of detachment from one’s surroundings.
Importantly, these effects were short-lived, generally resolving within hours of dosing. Safety monitoring also showed no increase in suicidal thinking compared to placebo, an encouraging finding in depression research. No treatment-related deaths were reported.
Still, the psychoactive nature of the drug meant some participants could guess whether they had received esketamine or placebo, a limitation that researchers acknowledged.
For the estimated 280 million people worldwide living with major depressive disorder, and especially the one-third who don’t respond to conventional drugs, esketamine represents a potential paradigm shift.
Traditional antidepressants work by altering serotonin, norepinephrine, or dopamine. Esketamine instead targets the glutamate system, offering a novel mechanism of action. That difference matters- before SPRAVATO, psychiatry hadn’t seen a new treatment pathway in over 30 years.
Adam Janik, medical director at Johnson & Johnson and lead author of the study, emphasized the scale of the problem: “The size and scope of the global depression epidemic is staggering. Significant unmet needs remain for these patients.”
While the findings are promising, experts caution against premature conclusions. The trial population was not racially diverse, and individuals with certain psychiatric conditions were excluded. That raises questions about how well the results will apply across broader patient groups.
For decades, patients with treatment-resistant depression have cycled through medications, therapies, and hospitalizations, often with little relief. This study adds weight to a growing body of evidence that esketamine could break that cycle. While it is not a cure, the possibility of meaningful relief within hours rather than weeks could be life-changing for millions.
Two Premature Infants Die From Infection In Italy Hospital Over Suspected Soap Dish Contamination
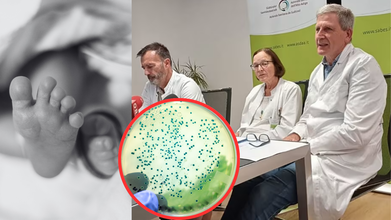
Credits: Canva/ South Tyrolean Health Service
Two of the premature infants, each weighing under two pounds, succumbed within days of one another at San Maurizio Hospital in Bolzano, Italy. The probable cause: Serratia marcescens, a bacterium traced to detergent dispensers used to wash baby bottles and teats in the neonatal intensive care unit (NICU).
The two premature infants, born at 23 and 27 weeks of gestation, died from overwhelming sepsis on August 12 and 13. Both deaths have raised serious questions about the hospital's infection control and hygiene practices, as well as the exceptional vulnerability of premature infants.
Italy's National Anti-Corruption and Prevention Agency (NAS) is spearheading the investigation into how a kitchen staple—dishwashing detergent—was used as a lethal vector. Preliminary tests isolated Serratia marcescens in the spout and dispensers utilized for the soap reservoir in the NICU. Authorities are investigating several alternatives:
- The detergent could have been contaminated during transport to the hospital.
- Improper storage or handling could have provided an environment for bacteria to flourish.
- An infected person may have transferred the pathogen through contact with the dispenser.
The route of contamination is uncertain until laboratory tests are done.
What Is Serratia marcescens?
Serratia marcescens is no stranger to hospitals. The bacteria exist best in damp surroundings—sinks, soap dispensers, catheters—and though usually harmless to healthy patients, are deadly to those with weakened immune systems. For preemies, whose immune systems are still developing, contact can rapidly degenerate into sepsis, pneumonia, or meningitis.
Hospital administrator Pierpaolo Bertoli emphasized that the germ itself was not novel. "The bacterium's presence is not singular because it is always a threat to neonatal intensive care units," he stated. The threat, he continued, is in the immense vulnerability of NICU patients.
Immediate Response and Precautionary Measures
After the discovery, a recall of all soap from the Bolzano hospital system was ordered, and admission of no more high-risk premature infants is taking place until notice. Existing cases are being routed to hospitals in Trento, about 40 miles away.
The hospital has even transferred the 10 remaining NICU babies to a different wing as a precautionary step. The hospital's medical director, Dr. Monika Zaebisch, confirmed that proper hygiene procedures were in effect but accepted the tragedy. "Unfortunately, these two cases could not be prevented," she stated.
Why Premature Babies Are Susceptible To Deadly Infections
Prematurity, or delivery prior to 37 weeks, leaves babies with a poorly developed immune system. Under normal circumstances, transfer of protective antibodies from the mother through the placenta in the third trimester supplies babies with at least partial immunity at birth. In preemies, that all-important exchange is incomplete—or nonexistent.
This absence of maternal immunity, coupled with medical interventions like catheters, ventilators, and feeding tubes, provides an entry point for bacteria such as Serratia marcescens. Infections that would be contained within older children or adults—such as bloodstream infections, pneumonia, or meningitis—can develop quickly and be life-threatening in preterm infants.
The Bolzano tragedy is no lone wolf. Hospitals around the globe have experienced outbreaks attributed to contaminated medical devices, water supply lines, and cleaning solutions. Even slight breaches of sterilization procedures can snowball into fatal infections in vulnerable wards such as NICUs.
Dr. Josef Widmann, director of medicine for the South Tyrolean Health Authority, recognized the systemwide problem. "This is not merely one product issue," he told a press briefing. "It is a reminder that NICUs remain under constant risk of microbial exposure.
The incident comes on the heels of another Italian public health crisis: a botulism outbreak from a food vendor in southwest Italy that resulted in two deaths and sent 14 to the hospital. Combined, the incidents highlighted systemic weaknesses in food and health safety regulation.
The Public Prosecutor of Bolzano is contemplating autopsies on the infants, which would determine whether charges of malpractice were appropriate. Relatives and health advocates are demanding answers, both to pay respects to those who died and to guarantee system change.
In the meantime, NAS researchers persist in testing the recalled detergent, dispenser units, and environmental swabs from the NICU. Identification of where the contamination took place, at the production facility, in storage, or within the hospital system itself, is important not only for legal accountability but also for avoiding future such outbreaks.
World’s First Pregnancy Robots: Will Women Soon Be Able to Use Surrogate Robots?

(Credit - Heathandme)
While technology has long assisted in the birthing process, researchers at Kaiwa Technology, China are now taking it a step further by creating artificial wombs that can be placed inside a robot. According to Dr Zhang Qifeng, who is leading the project, the technology for artificial wombs is already significantly advanced, and the team aims to demonstrate a working prototype as early as next year.
The robot is expected to ‘become’ pregnant and carry a baby to full term in an environment filled with artificial amniotic fluid, designed to mimic the natural conditions of a human womb. Although the exact process of fertilization has not yet been disclosed, the project is being described as a potential game-changer for people who cannot conceive naturally or are unable to carry a pregnancy to term.
Ethical Concerns And Unanswered Questions
As with any radical new technology, the robot womb comes with serious ethical and legal questions. How will the egg and sperm be fertilized? What does the birthing process look like? And who will be legally responsible for the baby? Dr Qifeng says discussions with Chinese officials are already underway to create new regulations for this emerging field.
One of the biggest draws as per the company is its cost. The estimated price of using the robot for surrogacy could be around $14,000, a fraction of the $100,000–$200,000 typically spent in the U.S. for human surrogacy.
New Era Of Reproduction Or Dystopian Step?
The news has sparked a heated debate. Supporters believe the technology could offer new hope to infertile couples and free women from the physical risks of pregnancy. Some even see it as a potential solution to China’s growing infertility crisis.
Critics believe that the biological and emotional complexity of human pregnancy cannot be replicated by a machine. Experts fear that growing a baby in a completely artificial environment could have unknown effects on the child’s health and well-being, and that removing the maternal bond from the process might lead to ethical dilemmas.
© 2024 Bennett, Coleman & Company Limited

