- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Antibody Vs Diagnostic: How To Know If You’re Taking The Right COVID-19 Test?
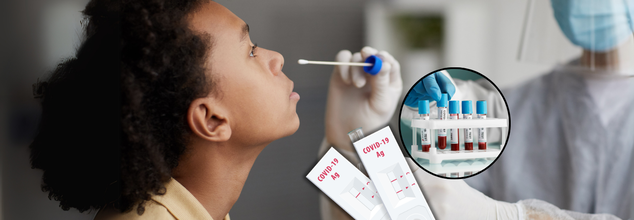
With the world still dealing with the aftermath and ongoing presence of COVID-19, there is one question more relevant than ever: What COVID-19 test is appropriate for me—diagnostic or antibody? If you are ill, exposed, or just wondering about your immunity, knowing how to distinguish between COVID-19 test types is the important step in informed decision-making for your own health and the health of others.
It has already been over five years since the world was initially attacked by the COVID-19 virus, which is rearing its head again in the form of new variants, primarily in the Asian nations, such as Singapore, Hong Kong, and Thailand. India also reported more than 1,000 active cases, while the new variant has spread to the US as well, killing more than 300 individuals, according to the reports.
Antibody testing, also known as serology testing, is designed to detect antibodies in your blood. Antibodies are proteins your immune system produces after exposure to pathogens such as viruses, including SARS-CoV-2—the virus that causes COVID-19.
This type of testing is not meant to diagnose a current COVID-19 infection. Rather, it identifies whether you’ve previously had the virus or developed antibodies as a result of vaccination. According to the CDC, antibody testing should be conducted 1 to 3 weeks after a suspected infection to allow time for your immune system to produce detectable antibody levels.
A positive antibody test may indicate previous exposure or vaccination but not necessarily immunity or protection against reinfection. Nor can it tell you if you are currently infectious or still carry the virus. The FDA has approved certain antibody tests, but not all tests are created equal.
When Are Antibody Tests Useful?
Antibody tests are usually given for the following reasons:
Post-infection diagnosis: If you believe you contracted COVID-19 but were never tested while the illness was active, an antibody test can confirm a past infection.
Eligibility to donate plasma: Individuals with high antibody levels can be eligible to donate convalescent plasma, which has been used to treat others with severe COVID-19.
Research into post-COVID complications: Doctors may employ antibody tests to aid in the diagnosis of unusual complications such as multisystem inflammatory syndrome, particularly in kids.
It's critical to point out that timing is an important factor. Getting tested too early after exposure or infection will provide a false-negative since the body hasn't had time to produce antibodies at the time of the test.
Role of Diagnostic Testing
Diagnostic tests are different from antibody tests in that they try to establish if you are currently infected with the virus. There are two major categories of diagnostic tests:
1. Molecular Tests (PCR/NAAT)
PCR tests identify the genetic material (RNA) of SARS-CoV-2, the virus that causes COVID-19. They rely on a very sensitive laboratory method called polymerase chain reaction (PCR) that makes a few viral RNA copies even if it is in small quantities for detection. High sensitivity is characteristic of PCR tests, which are the gold standard for detecting COVID-19. They are usually obtained by performing a nasal or throat swab, although saliva samples are sometimes utilized. Tests are available in as little as a few hours to a few days, depending on the testing lab. PCR testing is particularly useful within the first few days of infection, even at low viral loads. They are not, however, ideal for establishing when to discontinue isolation since they can still be detecting non-infectious viral fragments many weeks after recovery.
2. Antigen Tests (Rapid Tests)
Rapid antigen tests recognize certain proteins (antigens) on the surface of the virus and are widely used in rapid, home test kits. Although these tests provide immediate results in many cases within minutes—they are less sensitive than the PCR tests, particularly in the case of asymptomatic individuals. A sample is usually taken by a nasal or throat swab. Although less sensitive, rapid antigen tests prove to be very useful in certain situations like big gatherings, school or workplace screenings, and travel, where instant results are required. But if one turns out to be negative using an antigen test but still has symptoms, it is advised to retest within 48 hours or double-check using a more sensitive PCR test.
How To Choose the Right COVID-19 Test?
A number of factors determine which COVID-19 test you should undergo. If you have a fever, cough, or are tired, a diagnostic test—either PCR or antigen—is warranted to establish an active infection. In recent exposure with no symptoms, the CDC recommends testing five days later with a diagnostic test to detect possible asymptomatic infections. If you wonder whether you had COVID-19 weeks or even months ago, an antibody test might give you some information about past infection. For vaccinated individuals who need to test for immune response, antibody testing will show partially that you have antibodies, but it won't prove immunity. If you're traveling or going to a function, certain test types will be needed, so you'll need to look at the rules ahead of time.
How Accurate Are COVID-19 Tests?
PCR Tests: Very sensitive and highly accurate, they can pick up even tiny amounts of viral RNA. Taking the test too soon, though—before a sufficient amount of virus is in the system—may yield a false-negative.
Antigen Tests: Quick and inexpensive, but more likely to be false-negatives, particularly when taken too soon. To have better accuracy, repeat testing every 48 hours is advisable.
Antibody Tests: Timing and quality determine accuracy. Too early, antibodies may be missed, and not all tests are FDA-approved.
After COVID-19 When Should You Test Again?
If you have recovered from COVID-19 and wish to determine your immunity level, an antibody test can identify the level of IgM, IgG, or IgA antibodies in your blood. However, health professionals advise against concluding that a positive result on an antibody test is a guarantee of long-term protection.
In the U.S., organizations like the American Red Cross are still conducting antibody testing on donor blood to identify potential plasma donors. If you're interested in helping others post-recovery, this could be a way to contribute.
COVID-19 testing isn't one-size-fits-all. Knowing which test to take and when can make all the difference in getting accurate information and taking appropriate action.
If you have symptoms or have known exposure, have a diagnostic test. If you're interested in previous exposure or potential immunity, have an antibody test—but be realistic.
'It’s Not Too Late': Even After A Lung Cancer Diagnosis, This One Habit Change Can Save Your Life

Credits: Canva
A lung cancer diagnosis shatters worlds. It thrusts patients into a whirlwind of fear, confusion, and uncertainty. What now? How will I cope? Is this the end? These questions are inevitable and urgent. Yet, amid the anxiety, many patients harbor a dangerous belief: that if cancer has taken hold, quitting smoking is pointless.
It's not just a myth, it’s dangerous. Newer studies show quitting smoking even after a cancer diagnosis can significantly improve survival, make treatments more effective, and alleviate symptoms. In other words: even after cancer strikes, letting go of cigarettes can save your life.
In many small towns across India, where awareness about cancer and tobacco risks is limited, patients often continue to smoke despite their diagnosis. “Some feel it is too late to stop. Others are too addicted or too hopeless to try,” says Dr. Ruchi Singh, HOD & Senior Consultant of Radiation Oncology at Asian Hospital. This is the kind of thinking that kills from the inside out.
The reality is the opposite. Dr. Singh emphasizes, “We try to explain … it is never too late. If they stop smoking, even after the cancer has started, the treatment becomes more effective. It is one of the most important things they can do for themselves.”
Every cigarette after diagnosis undermines treatment, weakens the body, and shortens survival. But should someone quit even late into their cancer journey their lungs begin to heal, treatments work better, and recurrence becomes less likely.
How Does Quitting Smoking Extends or Saves Lives?
Global research aligns with Dr. Singh’s clinical advise, a study by IARC and Russian oncologists followed 517 lung cancer patients who smoked at diagnosis. Those who quit within three months lived 22 months longer on average and had 33% lower mortality risk and 30% lower disease progression, regardless of stage or smoking intensity.
The Prospective cohort of the Annals of Internal Medicine confirmed quitting after diagnosis yields meaningful survival benefits.
MUSC Hollings Cancer Center. A Harvard study of nearly 5,600 non-small cell lung cancer (NSCLC) patients found former smokers lived longer than current smokers, suggesting even pre-diagnosis quitting increases survival. Additional studies show quitting at or around diagnosis reduces mortality significantly and improves outcomes across all stages of NSCLC.
Smoking cessation isn’t just beneficial—it is one of the most powerful lifesaving interventions for lung cancer patients.
People tend to discount vaccines or preventive care because success makes the threat invisible. Lung cancer prevention has been a public health battle for decades. Policymakers and physicians worked to reduce smoking rates, and incidence fell. But once a cancer diagnosis arrives, all remission plans depend on a foundation of good habits—like quitting tobacco.
Tobacco smoke introduces toxins, weakens immune function, and diminishes treatment outcomes. Continuing to smoke after diagnosis:
- Lowers treatment efficacy
- Increases post-surgery complications
- Heightens the risk of recurrence or second primary cancers
- Shortens survival significantly
- Quitting reverses much of that risk—even post-diagnosis.
How to Quit Smoking After Lung Cancer Diagnosis?
Treatment regimens already overwhelm patients. Quitting smoking under stress and physical duress is tough—but not impossible. With the right support, patients dramatically increase their success odds. Here’s a compassionate roadmap:
- Set your quit date now, delay weakens motivation and clarity.
- Seek medical support, consult your oncologist or a specialist in Siliguri. Therapy, nicotine replacements, or counseling can double your quitting success.
- Lean on loved ones, share your commitment and ask for accountability.
- Identify your triggers- stress, discomfort, or routine can push you back. Replace smoking with walking, meditation, or tea.
- Shift lifestyle habits and replace “smoke with morning coffee” rituals with alternative rituals—like stretching or herbal tea.
- Build habits that support health- hydration, nutritious food, gentle movement and joy—these restore lung health and resilience.
Indeed, about 36% of tobacco-linked lung cancer patients manage to quit after diagnosis. Those are lives reclaimed.
Lung cancer still has a stigma. Many see it as self-inflicted. That stigma often delays help—including quitting support. But as Dr. Singh reminds us: “People think cancer means a death sentence. But many cases are treatable, especially if caught early. If someone quits smoking, we see real improvement such as better breathing, better recovery after surgery, and fewer chances of the cancer coming back.”
For patients, oncology teams, and families, smoking cessation after diagnosis isn’t optional—it’s urgent evidence-backed medicine.
A lung cancer diagnosis changes everything, but it does not define what comes next. Quitting smoking—even when the disease has already appeared—creates space for healing, response, and survival. It says, “I’m still here. I’m still fighting."
If you or someone you love is facing lung cancer- quit, today. It doesn’t erase the past—but it can extend the future. Numbers don’t lie: treatment plus quitting smoking can give us 22 more months, more energy, more peace, and a higher chance of beating this disease. Quitting is more than choice. It’s courage. And it is always worth it.
World Lung Cancer Day 2025: Is Pollution The New Cigarette For Your Lungs? 5 Habits To Save Your Breath

Credits: Health and me
When you stop for a moment, take a deep breath. How clean do you think is the air you just inhaled? If you live in a city or anywhere near heavy traffic, construction zones, or industrial areas chances are that breath carried more than just oxygen. Increasingly, health experts are raising a red flag: air pollution is becoming just as dangerous for your lungs as cigarette smoke. Unlike a lit cigarette, you can’t see it or smell it as easily, but the damage? It’s happening all the same—quietly, gradually, and across the globe.
On World Lung Cancer Day, it’s time to stop treating pollution as a background inconvenience and start seeing it for what it is: a major, modifiable threat to lung health.
We’ve long understood smoking as the leading cause of lung cancer, but the health narrative is shifting. Air pollution is catching up—and fast. According to the World Health Organization, over 7 million people die each year from air pollution, with billions more living with compromised respiratory health. Dr. Tedros Adhanom Ghebreyesus, Director-General of WHO, didn’t mince words back in 2018 when he said, “Air pollution is the new tobacco.”
The culprits are tiny, invisible particles like PM2.5 and PM10, nitrogen dioxide from vehicles, and toxins from burning biomass or fossil fuels. These pollutants don’t just irritate the lungs—they penetrate deep into lung tissue, triggering inflammation, reducing immune clearance, and increasing the risk of chronic respiratory illnesses and cancer. As the air gets dirtier, lung cancer in non-smokers—especially women and young adults—is on the rise.
Is Air Pollution Is the New Smoking?
We are already witnessing the consequences of environmental neglect warns Dr. Sachin Trivedi, Director of Medical Oncology at HCG ICS Khubchandani Cancer Centre, further adding, "Cigarette smoking has been known to be the major cause of lung cancer over the past decades. But there is a more recent and equally threatening danger that is on the rise: air pollution."
Dr. Trivedi highlights that a significant number of lung cancer diagnoses are now occurring in non-smokers, suggesting a stronger role for environmental pollutants. From vehicle emissions and industrial fumes to household fuel burning, the sources of this silent threat are everywhere. These pollutants infiltrate deep into the lungs, sparking chronic inflammation, oxidative stress, DNA damage, and even malignant mutations.
Why Lung Damage Due To Pollution Doesn’t Show Symptoms?
Even more concerning, these changes often don’t produce symptoms until the disease is advanced. Which is why early detection, lifestyle awareness, and pollution avoidance are critical. Dr. Trivedi urges individuals to recognize and act on subtle warning signs like chronic cough, shortness of breath, or unexplained weight loss, especially among non-smokers who may not suspect lung cancer.
5 Lung-Saving Habits You Can Practice Daily
Despite the scale of the problem, Dr. Trivedi emphasizes that it’s possible to shield your lungs through smart, consistent habits:
1. Wear a Protective Mask Outdoors
Especially in high-traffic or industrial areas, an N95 mask can block harmful particles like PM2.5. It’s a frontline defense your lungs will thank you for.
2. Maintain Clean Indoor Air
Ventilation is key. Use exhaust fans in kitchens, avoid indoor smoking, and install HEPA-filter air purifiers in high-pollution zones. Urban homes need this extra layer of air hygiene.
3. Limit Outdoor Time on High AQI Days
Track air quality through reliable apps. Skip rush hour outings and outdoor workouts when air quality is poor. Exposure control is protection.
4. Eat an Antioxidant-Rich, Lung-Friendly Diet
What you eat matters. A diet high in vitamins A, C, and E from foods like berries, citrus fruits, broccoli, and nuts can counter oxidative lung damage. Turmeric and green tea also offer anti-inflammatory benefits.
5. Don’t Dismiss Early Symptoms
A persistent cough or breathlessness isn’t always a passing cold. Get medical attention early—especially if you're a non-smoker experiencing unusual respiratory symptoms.
Dr. Devendra Parikh, Consultant in Surgical Oncology at HCG Aastha Cancer Centre, adds in a perspective, "Chronic polluted air harms our lungs just as smoking does: it silently, over time, injures delicate tissue and raises cancer risk. He stresses that fine particles from cooking smoke, traffic fumes, or even poorly ventilated homes carry microscopic toxins that inflame lung tissue and trigger genetic changes." He further shares more ways in which you can protect yourself
- Checking your local AQI each morning helps you make smarter decisions. When air quality dips, stay indoors and keep windows shut.
- These devices capture up to 99.97% of fine particles, drastically improving indoor air. Even running a unit for a few hours can ease respiratory strain.
- A proper N95 or KN95 mask creates a secure barrier against inhaling toxic particles. It’s essential during commutes or outdoor errands on high-smog days.
- Simple breathwork techniques or pranayama for five minutes daily can help clear the lungs and improve capacity.
- For those over 50 or with occupational exposure, low-dose CT scans can detect early signs of lung cancer—even before symptoms begin. Early action saves lives.
You can’t filter the world. But you can control your exposure, build resilient habits, and stay alert to what your lungs are telling you. The new reality is this: pollution is the new cigarette, and we can no longer afford to breathe without awareness. On World Lung Cancer Day and beyond—your breath is worth protecting. In cities where clean air isn’t guaranteed, your daily choices are your lungs’ best defense.
Pollution may feel as unavoidable as city noise, but it doesn’t have to be as destructive. By weaving these habits into your daily life, you give your lungs the best chance to clear toxins, reduce inflammation, and ward off the long-term threat of lung cancer.
PCOS Isn’t Just Hormonal, It Might Be Fueling This Dangerous Heart Condition

Credits: Canva
Polycystic Ovary Syndrome (PCOS) is often dismissed as a reproductive or cosmetic issue—a condition marked by irregular periods, acne, and weight fluctuations. But the hormonal and metabolic underpinnings of PCOS go much deeper. New findings presented at ENDO 2025, the annual meeting of the Endocrine Society, now highlight a troubling link: PCOS may significantly increase the risk of vascular events in women with thrombotic disease, and these events are occurring at increasingly younger ages.
The research, presented by a team from Riverside University Health System Medical Center and supported by national health data, suggests that the combination of PCOS and thrombotic disease creates a more dangerous cardiovascular profile, one that might be going under-recognized in clinical settings.
The analysis drew on records from the National Inpatient Sample (NIS), evaluating over 205,000 women hospitalized between 2016 and 2022 for thrombotic, atherosclerotic, or cerebrovascular diseases. The goal was to see how outcomes differed based on the presence or absence of comorbid PCOS. The findings were both significant and sobering:
Women with both PCOS and thrombotic disease had higher stroke rates (14.81%) compared to those without PCOS (11.91%).
A greater percentage of women with PCOS were under the age of 50 at the time of their vascular event compared to those without PCOS across all categories—thrombotic, atherosclerotic, and cerebrovascular.
Also Read: Your Home May Look Clean, But These 8 Spots Are Dirtier Than You Think
Despite the younger age of presentation, in-hospital mortality rates were similar between women with and without PCOS, suggesting the seriousness of these events in younger populations is not being offset by age-related resilience.
Dr. Alexander Lim, DO, who presented the findings, noted, “We found that cardiovascular events in women with PCOS were more likely to occur at an earlier age. The risk tends to decrease with age, possibly due to underdiagnosis of PCOS in older women or survivorship bias.”
Why Metabolic Difficulties Due To PCOS Lead Vascular Complications?
To understand why PCOS might elevate vascular risk, it's crucial to look at its underlying pathology. PCOS is not just a hormonal disorder—it’s also deeply metabolic.
Women with PCOS frequently exhibit insulin resistance, even if they are not overweight. This insulin resistance is a precursor to type 2 diabetes, hypertension, nonalcoholic fatty liver disease, and dyslipidemia—all conditions that significantly increase the risk of both arterial and venous thrombotic events.
Despite this, clinical management of PCOS often remains centered on cosmetic symptoms (like acne and hirsutism) or fertility concerns, rather than addressing the long-term metabolic and cardiovascular consequences. This treatment gap may explain why vascular events in women with PCOS catch both patients and providers off guard.
Why Current Testing May Be Failing Women?
Another striking aspect of the new research is that women with PCOS who experienced recurrent thrombotic events (strokes, heart attacks, pulmonary embolisms) often showed normal results in standard coagulation tests. This was identified in a diagnostic review by a coagulation management team and later verified through a national data set analysis using TriNetX, a large health research network.
Between 2013 and 2018, researchers used the TriNetX platform to analyze PCOS patients aged 15–75, excluding those with known thrombotic disorders, smoking history, or HIV. They found that thrombotic events occurred at significantly higher rates in women with PCOS compared to matched controls, even though clinical tests failed to detect abnormalities in coagulation.
What this suggests is that standard coagulation assays may not be sensitive enough to detect the unique thrombotic risk profile in PCOS, raising concerns about diagnostic error or delayed interventions.
This is the first large-scale study to systematically evaluate the rate and clinical presentation of thrombotic events in women with PCOS using national data and validated diagnostic algorithms like SPADE (Symptom-Disease Pair Analysis of Diagnostic Error).
By comparing outcomes 90 days prior to major thrombotic events, the researchers identified consistent symptom patterns—including subtle indicators—that could serve as early warning signs for intervention. However, the precise biological mechanisms behind thrombosis in PCOS remain poorly understood.
The message is clear: PCOS isn’t just a reproductive disorder—it’s a systemic, vascular-risk condition that can dramatically impact a woman’s health long before menopause. Clinicians need to think beyond fertility and skin health and recognize that young women with PCOS may be walking around with unrecognized cardiovascular vulnerabilities.
For women living with PCOS, this means that a heart-healthy lifestyle—including regular cardiovascular screening, metabolic management, and awareness of symptoms like chest pain, migraines, and swelling—needs to be part of long-term care, not just pregnancy planning.
© 2024 Bennett, Coleman & Company Limited

