- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
What Happens To Your Body When You Eat Raspberries

Credit: Canva
Sweet, tart, and vibrantly red, raspberries are more than just a delicious fruit—they're a nutritional powerhouse. Their soft, slightly fuzzy texture and naturally refreshing taste make them a great addition to smoothies, yoghurt bowls, oatmeal, and salads. But what makes raspberries truly stand out is their impressive health profile. Rich in fiber, antioxidants, and vitamin C, these berries offer multiple benefits for your heart, digestion, blood sugar, and immune system.
Gut Health and Regular Digestion
One standout feature of raspberries is their fibre content. Just one cup delivers 8 grams of fibre, nearly a third of the recommended daily intake for women. “This high fiber content promotes healthy digestion and regular bowel movements,” says Elizabeth Harris, a registered dietitian. Raspberries are especially rich in insoluble fiber, which adds bulk to stool and helps it move more easily through the digestive tract. This can reduce the risk of constipation and support overall gut health.
A Heart-Healthy Snack
Raspberries also support cardiovascular health. While their vitamin C and fibre content are well-known, they also offer potassium, a mineral that plays a key role in managing blood pressure. Research has shown that regular consumption of raspberries may improve cholesterol levels, especially in individuals with high cholesterol or metabolic syndrome. These effects are likely linked to the berry’s mix of fiber and polyphenols, plant compounds known for their antioxidant properties.
Balanced Blood Sugar Levels
For people with diabetes or prediabetes, raspberries are an ideal fruit. They’re low in sugar and high in fibre, which helps prevent spikes in blood glucose levels. According to the American Diabetes Association, berries are one of the healthiest carbohydrate sources. When paired with protein or healthy fats like Greek yogurt or nuts, raspberries become a satisfying, blood sugar-friendly snack.
Fighting Inflammation With Antioxidants
Raspberries contain powerful antioxidants, including anthocyanins and polyphenols, which help combat inflammation and oxidative stress in the body. “These compounds can lower the risk of chronic conditions like heart disease and cancer,” Harris explains. Their anti-inflammatory properties also make raspberries a key component of the MIND diet, which supports brain health and may slow cognitive decline.
Immunity Boost With Vitamin C
With over a third of your daily vitamin C needs in just one cup, raspberries can help keep your immune system strong. Vitamin C supports wound healing, protects cells from free radical damage, and strengthens your body’s defence against infections.
Tips for Storage And Enjoyment
To keep raspberries fresh, store them in an airtight container lined with paper towels and refrigerate. Wait to rinse until you’re ready to eat. For longer storage, freeze them in a single layer before transferring to a sealed bag.
Whether eaten plain, swirled into chia seed jam, blended into smoothies, or sprinkled on cereal, raspberries are a versatile and nutrient-rich fruit worth making a regular part of your diet.
What Really Happens In Your Brain During Deep Sleep

Credits: CANVA
Sleep takes up nearly one-third of a person’s life, yet many still wonder what really happens while we rest. Until the mid-20th century, scientists believed sleep was simply a time when the body and brain shut down.
Research now shows that sleep is far from passive, it’s an active, restorative process essential for mental and physical health. As Johns Hopkins neurologist and sleep expert Dr. Mark Wu explains, the brain remains deeply engaged during sleep, performing vital tasks that influence memory, mood, and overall well-being.
The Stages of Sleep
Experts say the human sleep cycle has four main stages that repeat throughout the night. The first three make up non-rapid eye movement (non-REM) sleep, while the fourth is REM sleep, the stage most closely linked with dreaming.
In the first stage of non-REM sleep, the brain and body begin to shift from wakefulness to rest. Brain activity slows, muscles relax, and it is common to experience small, sudden twitches.
During the second stage, the body’s temperature drops slightly, and breathing and heart rate slow. Brainwaves continue to decelerate, though quick bursts of activity may still appear as the brain processes and stores information.
The third stage marks deep sleep, which is the most restorative phase. Here, the body fully relaxes, and the heart rate, breathing, and brain activity reach their lowest levels. This stage is crucial for waking up feeling refreshed and for healing and repair processes throughout the body.
The final stage is REM sleep, which begins about 90 minutes after you fall asleep. It starts short, roughly 10 minutes, but lasts longer with each cycle. During REM, the eyes move rapidly beneath the eyelids, breathing quickens, and heart rate and blood pressure rise to near waking levels. This is when most dreaming occurs. Interestingly, as people age, the amount of REM sleep they experience gradually decreases.
How The Body Regulates Sleep
According to Dr. Wu, two main forces govern sleep: the circadian rhythm and the body’s sleep drive.
The circadian rhythm acts as the body’s internal clock, controlled by a cluster of brain cells that respond to light and darkness. This rhythm triggers the release of melatonin at night and halts it when morning light appears. People who are completely blind often struggle with sleep because their brains can’t register these light cues properly, as per the John Hopkins Study.
The sleep drive works much like hunger. The longer you stay awake, the stronger your urge to sleep becomes. Unlike hunger, though, your body can override your willpower, if exhaustion sets in, it can force sleep to happen, even during daily activities or while driving. In extreme fatigue, brief “microsleep” moments lasting just a few seconds can occur without a person realizing it. However, taking long naps later in the day can reduce this natural sleep pressure, making it harder to fall asleep at night.
Why Sleep Matters For Your Brain
Anyone who has felt mentally sluggish after a sleepless night knows how strongly rest affects the brain. Adequate sleep is key to brain plasticity—the ability to learn, adapt, and form memories. Without it, the brain struggles to retain new information and perform cognitive tasks. Scientists also believe that deep sleep allows the brain to clear out toxins that build up during waking hours, improving long-term brain health.
Sleep impacts far more than the mind. Poor sleep can worsen conditions like depression, high blood pressure, migraines, and even seizures. It weakens the immune system, leaving the body more vulnerable to infection. Metabolism also suffers, as just one night without enough rest can temporarily throw the body into a prediabetic state.
As Dr. Wu explains, “There are countless ways sleep supports health.” From mental clarity to physical repair, the hours we spend asleep are some of the most important for keeping the body and brain functioning at their best.
Can A Rash Be A Sign Of COVID-19? Here’s Everything You Should Know
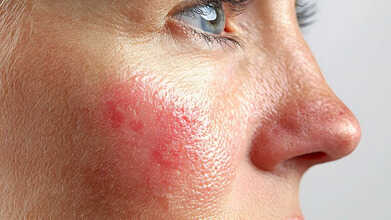
Credits: canva
When COVID-19 first emerged, it was largely seen as a respiratory illness. Over time, doctors discovered that the virus can affect nearly every major organ, including the heart, kidneys, liver, and skin. While cough, fever, and fatigue remain common signs, some people also develop unusual skin reactions. So, can COVID-19 actually lead to rashes? Here’s what experts have found.
Can COVID-19 Really Cause Rashes?
When COVID-19 first emerged, it was largely seen as a respiratory illness. Over time, doctors discovered that the virus can affect nearly every major organ, including the heart, kidneys, liver, and skin. While cough, fever, and fatigue remain common signs, some people also develop unusual skin reactions. So, can COVID-19 actually lead to rashes? Here’s what experts have found.
What Do COVID-19 Rashes Look Like?
Skin changes linked to COVID-19 are not among the most common symptoms, but they do occur. These rashes may appear on the neck, mouth, or toes and are often caused by inflammation in the body, as per Health website. They can look like flat or raised patches, small round spots, or itchy bumps. In some people, these rashes appear while they’re infected; in others, they show up weeks later.
Why Do These Rashes Occur?
Researchers believe COVID-related rashes are connected to how the virus interacts with the body’s ACE2 receptors, which are found in the skin. When the virus attaches to these receptors, it can trigger the release of inflammatory proteins called cytokines. This inflammation may lead to skin irritation, itchiness, or lesions.
Common Types of COVID-19 Rashes
1. COVID Toes
One of the most recognized skin signs of the virus, “COVID toes,” resembles chilblains, which are cold-weather sores. They appear as pink, red, or purple patches, sometimes with swelling or blistering. This condition is seen more often in younger people and may occur even after other symptoms fade.
2. Hives (Urticaria)
Hives tend to appear suddenly and can spread across any part of the body. They’re itchy, raised, and may come and go within hours or days.
3. Neck Eczema
Some people develop eczema-like rashes on the neck, chest, or trunk during or after COVID-19. The patches can be itchy and vary in color depending on skin tone—pink on lighter skin and brown, gray, or purple on darker skin.
4. Oral Rash
COVID can also cause soreness or peeling inside the mouth or on the lips. The area may feel dry, irritated, or scaly as it heals.
5. Vesicular and Papular Rash
These small, itchy bumps can be filled with fluid (vesicular) or solid (papular). They may appear anywhere on the body and are often linked with ongoing inflammation.
6. Pityriasis Rosea
This condition begins with a single large patch on the chest, back, or abdomen, followed by smaller spots that form a tree-like pattern. Though harmless, it can take several weeks or months to fade.
7. Purpuric or Vasculitic Rash
These rashes appear as dark, bruise-like spots caused by small blood vessel damage under the skin. The color may range from red and purple to brown or black, depending on skin tone.
How Long Do COVID Rashes Last?
The duration depends on the type of rash and the person’s immune response. Most clear up within 2 to 12 days, but some, especially in long COVID cases, may persist for weeks.
How Are COVID Rashes Treated?
Many rashes resolve without any special treatment. To relieve itching or pain, applying mild hydrocortisone cream can help. For more severe or persistent cases, doctors may recommend:
- Antihistamines to reduce itching
- Corticosteroids to lower inflammation
- Blood thinners if the rash is linked to blood vessel irritation
Your doctor will determine the safest treatment depending on the type of rash and overall health.
Disclaimer: This article is for general informational purposes only and should not be considered medical advice. Always consult a qualified healthcare professional before starting, stopping, or changing any medication, or if you experience any unusual symptoms or side effects.
Don't Ignore Your 'Winter Blues', This Is The Biological Reason Behind Winter Depression

(Credit-Canva)
It is a common theme for people to feel down and sad in winter. However, why do shorter, colder days often bring on feelings of loneliness and gloom? There is a biological reason behind it. As the days get shorter, many people feel a dip in energy or mood, but for millions, this signals Seasonal Affective Disorder (SAD), which is a serious form of depression that shouldn't be ignored. Experts from West Virginia University (WVU) caution that SAD symptoms are very similar to major depressive disorder and must be taken seriously.
What Causes SAD?
SAD is much more than just feeling down when it gets dark. It's a genuine type of depression most often seen during the winter months, especially in places far north where daylight is scarce. The basic problem is simple: less natural light hits your eyes. This drop in sunlight confuses your brain's chemistry.
It messes with two vital brain chemicals: serotonin, which helps stabilize your mood, and melatonin, which controls when you sleep and wake up. This lack of light also throws off your body's internal clock, called the circadian rhythm. When all these elements get disrupted, it triggers feelings of low energy and depression.
What Are The Symptoms Of SAD?
SAD involves a cluster of symptoms that persist and significantly interfere with your daily life. The pattern is usually predictable: symptoms begin in the fall, peak in the winter, and disappear by spring. Symptoms often include:
- Feelings of hopelessness.
- Loss of interest in activities you once enjoyed.
- Significant changes in sleep (often sleeping more, or having disrupted sleep).
- Changes in appetite or increased food cravings.
- Difficulty concentrating and persistent fatigue.
The risk of SAD is higher among younger people, women, and those with a family history of mood disorders.
Can You Treat/Prevent Seasonal Affective Disorder?
Because Seasonal Affective Disorder is highly predictable, experts advise starting preventative treatments early in the fall. Seeing a healthcare provider is essential to determine the best plan and timing for treatment, rather than waiting for severe symptoms to appear later in winter.
Light Therapy
This involves sitting daily before a special light box emitting 10,000 lux of bright white light. Doing this for 30 to 60 minutes each morning is the most common and effective treatment for SAD, as it helps correct the imbalance caused by reduced sunlight exposure.
Cognitive-Behavioral Therapy (CBT)
This form of talk therapy teaches you practical skills to manage negative thinking patterns and behaviors linked to depression. CBT helps individuals reframe their outlook on winter and build effective coping mechanisms to reduce the impact of SAD symptoms.
Medication
In certain situations, a healthcare provider may prescribe antidepressant medication to help regulate mood-affecting brain chemicals like serotonin. This is often considered alongside light therapy or counseling, especially if symptoms of depression are severe or persistent.
Lifestyle Changes
Simple daily habits are powerful tools. Regular exercise boosts mood and energy, while maintaining a consistent sleep schedule keeps your body's internal clock stable. These practical steps offer significant support alongside clinical treatments.
© 2024 Bennett, Coleman & Company Limited

