- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
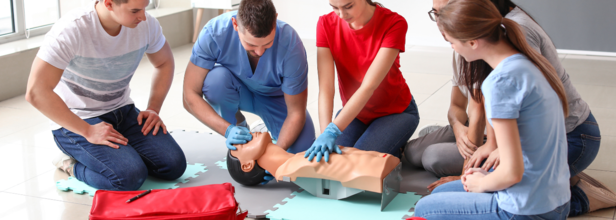
A Step-by-step Guide To Perform CPR Right Way
Credits: Canva
Before we get into how to correctly perform CPR, it is important to know what CPR is?
CPR stands for Cardiopulmonary Resuscitation, it is a crucial life-saving technique, which is used when someone stops breathing or their heart stops beating. This required acting quickly as it can double or even triple the person’s chances of survival.
This step-by-step guide lays down guidelines to perform CPR on people across ages including children, adults and the elderly.
The question is when would you know that it is the right time to perform CPR? For adults, if they are not responsive or are not breathing, it might be the right time to perform CPR. Same is the case for everyone else. However, you must avoid performing CPR if the person is just unconscious but is breathing. One must never perform CPR on a person who is conscious and breathing. It is important to also allow them the space once they gain back their conscious so they can breathe and gasp as much air as needed.
How to prepare for a CPR?
Step 1: it is always about ensuring safety
Check your surroundings for dangers, is there traffic? Is there a chance of fire or objects falling? If yes, try to reach and take the person to a safe place.
Step 2: Position the Person and Open the Airway
Place the person on their back on a firm surface. Now, tilt the person's head back slightly and lift the chin up to open the airway. It is important to do so to allow the air in. Look inside their mouth for any obstruction before performing CPR. If you see any object, carefully remove it.
Step 3: Check for Breathing
Listen for breathing sounds for no more than 10 seconds, if there are no beats, then perform CPR.
Performing CPR on Adults
Step 1: Chest Compressions
Place the heel of one hand on the center of the chest, slightly below the nipple line. Place your other hand on top and interlock your fingers.
Keep your elbows straight and push hard and fast—around 2 inches deep—at a rate of 100–120 compressions per minute.
Allow the chest to rise fully between compressions.
Step 2: Rescue Breaths
After 30 compressions, tilt their head back, lift the chin, pinch their nose, and seal your mouth over theirs.
Give two rescue breaths, each lasting about 1 second, watching for chest rise.
If the chest doesn’t rise, reposition the head and try again.
Step 3: Repeat the Cycle
Continue alternating 30 chest compressions and 2 rescue breaths until the person starts breathing or professional help arrives.
Performing CPR on Children and Infants
Step 1: Check Responsiveness and Provide Immediate Care
For children, tap the shoulder; for infants, gently flick the sole of the foot to check for a response.
If unresponsive and alone, provide 2 minutes of CPR before seeking emergency help.
Step 2: Adjust Technique for Size
For children: Use one hand for chest compressions, pressing down 2 inches (or one-third of the chest’s depth).
For infants: Use two fingers in the center of the chest, pressing down 1.5 inches.
Step 3: Rescue Breaths
For children, pinch the nose and cover their mouth with yours to give two breaths.
For infants, cover both the nose and mouth with your mouth and deliver gentle breaths.
Step 4: Continue CPR
Repeat cycles of 30 compressions and 2 breaths until the child or infant begins breathing or professional help arrives.
Key Considerations for CPR
Use CPR for emergencies like cardiac arrest, drowning, choking, or trauma.
Always ensure the person is unresponsive and not breathing before starting CPR.
Be prepared to adjust techniques for infants, children, and adults based on their size.
Note: this is only for emergency circumstance. If there is a healthcare or a medical professional next to you, it is always advisable to seek for their help.
FDA Issues Urgent Recall on Frozen Vegetable for Listeria—Here Are the Products You Should Avoid

Credits: canva
Hellofresh: As the listeria outbreak continues to spread across the United States, more products are being identified as contaminated. The U.S. Food and Drug Administration (FDA) has issued a recall for certain frozen spinach items found to contain Listeria monocytogenes. The affected spinach was sold under two names, Del Mar 35-pound Bulk Organic Frozen Spinach and Sno-Pac Organic Frozen Cut Spinach in 10-ounce packs, distributed to consumers across the country.
Each year, millions of Americans deal with food sensitivities and allergies. According to the FDA, the nine main food allergens in the United States include eggs, milk, fish, wheat, soybeans, crustacean shellfish, sesame, tree nuts, and peanuts.
HelloFresh Meals Contaminated With Listeria
Federal health officials have advised the public not to eat certain HelloFresh subscription meals containing spinach that may be contaminated with listeria. In connection with the same concern, Sno Pac Foods, Inc. has also recalled both its Sno Pac and Del Mar brands of organic frozen spinach products due to fears of Listeria monocytogenes contamination.
The recalled Del Mar Bulk Organic Frozen Spinach was sold in 35-pound boxes with an expiration date of January 1, 2027, and the following lot numbers: 250107A, 250107B, 250107C, 250107D, 2501071, and 2501073.
The Sno Pac Organic Frozen Cut Spinach came in 10-ounce containers with these lot codes and “best by” dates: SPM1.190.5 (best by July 9, 2027), SPC1.160.5 (best by June 9, 2027), SPC2.160.5 (best by June 9, 2027), and SPM1.097.5 (best by April 7, 2027).
The official notice warns: “Healthy individuals may experience short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain, and diarrhea. However, Listeria infection can lead to miscarriages and stillbirths in pregnant women.”
For more details, consumers can view the recalled product labels on the FDA website. Anyone who has these frozen spinach products at home is urged not to eat them. Instead, they should dispose of the items safely or return them to the store for a full refund.
Previous Listeria Outbreaks
Just last month, FreshRealm confirmed that tests showed pasta used in certain linguine dishes sold at Walmart carried the same strain of listeria linked to an earlier outbreak in June. That outbreak, which initially involved chicken fettuccine Alfredo, resulted in at least four deaths and 20 reported illnesses, the most recent occurring on September 11.
What Is Listeria Monocytogenes?
According to the National Institutes of Health, Listeria monocytogenes is a type of bacteria that causes the infection known as listeriosis. It is a facultative anaerobic organism, meaning it can survive with or without oxygen. The bacterium can live and multiply inside the body’s cells, making it one of the most dangerous foodborne pathogens known.
Listeria Symptoms
A listeria infection can be especially serious for older adults, pregnant women and their newborns, and individuals with weakened immune systems. Common symptoms include fever, muscle aches, headache, stiff neck, confusion, loss of balance, and convulsions.
The Centers for Disease Control and Prevention (CDC) estimates that around 1,600 people become ill from listeria infections each year, and roughly 260 die. Federal agencies said in December that they were revising safety procedures to prevent further outbreaks following several major incidents, including one linked to Boar’s Head deli meats that caused 10 deaths and more than 60 illnesses last year.
Supreme Court Rules Couples Who Started Surrogacy Before 2021 Law Can Proceed Despite Age Limits

Credits: Canva
The Supreme Court on Thursday ruled that the age limits under the Surrogacy (Regulation) Act of 2021 would not apply to couples who had already frozen their embryos before the Act came into force on January 25, 2022, according to a LiveLaw report.
The court heard petitions from three couples challenging the law’s age restrictions, 23 to 50 years for women and 26 to 55 years for men arguing that these limits should not apply to them since they had preserved their gametes long before the Act became effective.
What Does The Current Surrogacy Law State?
India’s current surrogacy law allows only altruistic surrogacy while banning commercial arrangements. In simple words, altruistic surrogacy involves no monetary payment to the surrogate. Typically, the surrogate is a close family member or friend of the intended parents.
Under the law, Indian married couples, as well as NRIs and OCI cardholders, can opt for surrogacy if they are medically infertile and meet the age and marriage criteria.
Key provisions include:
- Only altruistic surrogacy is permitted.
- Commercial surrogacy is completely banned.
- Eligible couples include Indian, NRI, and OCI married couples.
- Couples must be infertile and married for at least five years.
- Single parents, foreigners, and LGBTQ+ couples are not allowed.
- Surrogates must be a close relative, aged 25–35, and married.
- Surrogates must have at least one biological child.
- Mandatory medical and psychological screening is required.
- Health insurance coverage is required for 36 months after delivery.
- All procedures are overseen by a District Medical Board.
Age Bar Won’t Affect Couples Who Began Process Before 2021 Surrogacy Act
The petitions were filed through infertility specialist Arun Muthuvel, challenging the age-related amendments in the 2021 law. A bench comprising Justices B.V. Nagarathna and K.V. Viswanathan accepted the plea, stating:
“If the couple had commenced the surrogacy process before January 25, 2022, and were at the stage of embryo freezing after extraction and before transfer to the surrogate, the age restriction under Section 4(iii)(c)(1) of the Act will not apply in these cases,” as per Live Law.
The court added that, although only three couples had approached it, other couples in similar situations could also approach high courts to seek the benefits of this ruling. The court clarified that the judgment does not question the validity of the age bar or the law itself.
Before the 2021 law, there were no age restrictions, and these couples were within the legally acceptable age range. Having frozen their embryos years earlier, they would have otherwise been unable to proceed with surrogacy due to the new age limits.
The Union government opposed the petitions, arguing that age limits protect the welfare of the child, since older parents may not be able to meet the child’s needs. It maintained that the protection should apply only when the embryo is implanted in the surrogate, not at the embryo freezing stage.
The court rejected this argument, noting that the decision to have a child is a personal choice of the couple and involves no third party. “The surrogacy process is considered to have commenced once the couple extracts gametes and freezes the embryo. At this stage, the couple has already expressed their intent to pursue surrogacy, the only remaining step is the involvement of the surrogate mother,” the bench explained.
RFK Jr. Claims Tylenol After Circumcision May Be Linked to Autism

(Credit-Ben Curtis/AP & Canva)
Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. recently made a public statement, claiming there might be a connection between early circumcision and an increased risk of autism.
He believes this link is due to the common practice of giving infants Tylenol, also known as acetaminophen, for pain relief after the surgical procedure. Speaking to President Trump during a cabinet meeting, Kennedy cited studies suggesting that "children who are circumcised early have double the rate of autism," and he believes this is "highly likely, because they were given Tylenol." This is a significant claim from a top health official, directly questioning standard medical practices.
Also Read: Tramadol, Common Painkiller Found Ineffective For Chronic Pain, May Trigger THIS Serious Health Risk
Can Circumcision Cause Autism?
While RFK Jr did not specify what study made this link, a similar 2015 study has been highlighted by media reports, which could be the study that is being referenced. Published in the Journal of the Royal Society Medicine, 2015, this study from Denmark tracked over 340,000 young boys and found that males who were circumcised were more likely to be diagnosed with autism before they turned 10 compared to those who were not.
The researchers proposed that the pain and stress of the surgery early in life could potentially increase the risk for later problems with brain and psychological development.
However, this study was criticized for the result it came to, with experts refuting the case.
Are Their Studies That Refute ‘Circumcision And Autism’ Link?
Published in the same journal, a 2015 review questioned the validity of the results. They found a very slight, statistically shaky link for ASD in boys aged 0–4. This link was only seen in a small group of 28 Muslim boys who were circumcised before age 2. Due to the statistics, this link really only applied to about 10 boys in the entire study. Among non-Muslim boys aged 0-4 who were circumcised, they noted only six ASD diagnoses.
Furthermore, the data about Muslim boys with ASD seemed wrong. Of the 337 Muslim boys with ASD in the study, the records suggested that only 10.9% were circumcised, with the rest being uncircumcised. Critics say this number is highly unlikely to be accurate and makes the overall findings untrustworthy.
Another explanation for the finding is that boys who are circumcised have more visits with healthcare workers, which could simply mean autism is detected more often in this group, not that the procedure caused it.
Why Only Look at Circumcision Pain?
The authors of the Denmark study suggested that the link to autism was due to the pain of the circumcision procedure. Yet, critics point out a major flaw: if pain is the cause, why didn't the researchers look at other common, painful conditions?
For example, urinary tract infections (UTIs) cause severe pain and are much more common in uncircumcised infants and boys. If the researchers' "pain hypothesis" were correct, then UTIs, and therefore being uncircumcised, should have been linked to a higher rate of ASD. Since they didn't examine this, their focus on circumcision pain is incomplete.
The review concluded that the most likely explanation is that both an early ASD diagnosis and the decision to have an early circumcision are more likely to reflect parental conscientiousness—meaning parents who are more proactive, attentive, and engaged in their child's health and development may seek both the procedure and early developmental screenings.
Why Was Autism Linked To Circumcision Through Tylenol?
This claim has come after the recent announcement by the Trump administration. Announcements were made claiming that Tylenol (known as paracetamol in Australia and other countries) is linked to autism in babies when taken by pregnant women. He strongly suggested that women should "fight like hell" to avoid taking the medication.
Trump told women that continuing to take the medicine means "you can't tough it out," but ultimately it's "up to you and your doctor." His firm advice was clear: "don't take Tylenol. Don't take it. Fight like hell not to take it."
Trump's statements have sparked a strong disagreement among medical professionals. Health experts have stated that the alleged link between Tylenol use during pregnancy and autism is not supported by scientific evidence.
© 2024 Bennett, Coleman & Company Limited

