- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Alzheimer’s Risk Can Be Detected In 3 Minutes With At-Home Brainwave Test, How It Spots Early Memory Decline

Credits: Brace Dementia Research/ University Of Bath
Alzheimer’s disease has long been one of medicine’s most difficult puzzles. By the time symptoms such as memory loss, confusion, and language difficulties become obvious enough for a clinical diagnosis, the brain has often been undergoing silent changes for decades. Researchers have been urgently searching for ways to close this gap between disease onset and diagnosis. Now, a portable brainwave test called Fastball EEG is raising hopes that Alzheimer’s can be detected years earlier potentially transforming both treatment and quality of life.
Fastball EEG is a deceptively simple tool. The test takes just three minutes and involves wearing small sensors on the scalp while watching a stream of images flash across a screen. Unlike traditional memory exams that rely on a patient recalling lists or following instructions, this test is entirely passive. It works by measuring the brain’s automatic electrical responses when it sees something familiar.
Dr George Stothart, a cognitive neuroscientist at the University of Bath who led the study, explained the significance: “We’re missing the first 10 to 20 years of Alzheimer’s with current diagnostic tools. Fastball offers a way to change that—detecting memory decline far earlier and more objectively, using a quick and passive test.”
This early detection matters. Alzheimer’s develops silently, with proteins such as amyloid and tau building up in the brain long before memory loss is apparent. The earlier these changes are picked up, the more effective new treatments can be.
Detecting Early Cognitive Decline
The University of Bath trial evaluated Fastball in 107 participants: 53 people with mild cognitive impairment (MCI)—a condition often considered a precursor to dementia—and 54 healthy older adults.
MCI doesn’t always progress to Alzheimer’s, but when memory loss is its primary symptom, the risk rises sharply. Researchers found that people with amnestic MCI, the form most closely linked to Alzheimer’s, showed significantly weaker brainwave responses during the test compared to healthy volunteers or those with non-amnestic MCI.
In other words, the Fastball test was able to pick up the subtle brain changes that predict future decline, even when symptoms were not yet disabling. When participants were retested a year later, the results proved reliable, particularly among healthy adults, suggesting the test could track changes over time.
Why Bringing Testing Out of Clinics and Into Homes is Essential?
One of the most striking findings is that Fastball can be done at home. Traditionally, brain scans or memory assessments require in-person visits to specialist clinics. For many patients, these appointments are intimidating, costly, or simply inaccessible. By contrast, Fastball is portable, low-cost, and anxiety-free.
“All of the tests were performed in people’s homes, which is important for making them accessible and reducing people’s anxiety,” Dr Stothart said.
The potential here is enormous: routine memory screening in GP surgeries, community clinics, or even at home could become a reality. That shift could reduce the number of people living with undiagnosed dementia—a figure that remains stubbornly high worldwide. In the UK alone, the Alzheimer’s Society estimates nearly one in three people with dementia never receives a formal diagnosis.
Why Early Diagnosis is Important for Alzheimer’s?
Until recently, early diagnosis was often criticized as offering little benefit. After all, without effective treatments, what could patients do with the information? That calculation has changed with the arrival of new Alzheimer’s drugs such as donanemab and lecanemab.
These antibody therapies target amyloid, the sticky protein that clumps in the brains of people with Alzheimer’s. Clinical trials have shown they can slow cognitive decline—but only when given in the disease’s earliest stages. The challenge, then, is finding patients early enough to qualify.
As Dr Julia Dudley of Alzheimer’s Research UK explained, “New Alzheimer’s treatments are proving to be more effective when given at earlier stages in the disease, therefore earlier diagnosis is key for people to benefit from this.”
Fastball could be the missing link that connects patients to treatment while it can still make a difference.
While the promise is clear, experts caution against viewing Fastball as a standalone diagnostic tool just yet. Professor Sir John Hardy of the UK Dementia Research Institute noted that the test cannot distinguish Alzheimer’s from other types of cognitive decline. Biomarker tests—such as spinal fluid analysis or advanced imaging—will still be needed to confirm a diagnosis.
Prof Vladimir Litvak of UCL’s Queen Square Institute of Neurology described the study as “an early step towards developing a clinically useful test,” highlighting the need for larger, longer-term trials to confirm predictive power.
There’s also the question of diversity. Much of the early research has been conducted in relatively small, homogenous groups. For the tool to be globally useful, it must be validated across varied populations, including people with different ethnic backgrounds, education levels, and coexisting health conditions.
Dr Dudley emphasized that memory impairment is not always caused by dementia—it can also be linked to thyroid disease, depression, or even side effects from medication. Future studies, she said, must explore how these factors influence Fastball’s results and how the test might complement, rather than replace, existing diagnostic tools.
The stakes could not be higher. Dementia currently affects an estimated 55 million people worldwide, a number projected to triple by 2050 as populations age. Alzheimer’s disease is the most common cause, accounting for up to 70 percent of cases.
In the UK, nearly one million people are living with dementia, with that figure expected to rise to 1.4 million by 2040. In the US, the Alzheimer’s Association estimates more than 6.7 million Americans aged 65 and older are currently living with the disease. The global cost of dementia already exceeds $1.3 trillion annually, straining families, health systems, and economies.
Against this backdrop, a quick, inexpensive screening tool that can be deployed widely could be game-changing—not just for individuals and families, but for public health systems grappling with rising demand.
Chris Williams, chief executive of the dementia research charity BRACE, which supported the project, sees Fastball as a milestone. “Fastball is an incredible tool that could offer anyone who, for whatever reason, cannot access a dementia diagnosis in a clinical setting,” he said.
For now, Fastball remains experimental, but the momentum is clear. With further validation, it could become part of standard memory screening within the decade. For patients, it could mean earlier access to life-changing drugs. For families, it could mean more time to plan and adapt. And for medicine, it represents a shift toward proactive, rather than reactive, dementia care.
Alzheimer’s may still be incurable, but with tools like Fastball, the way we detect and manage it may be about to change dramatically.
The Next Ozempic? New 4-in-1 Weight Loss Drug Could Treat Obesity, Diabetes, Cancer And Heart Diseases

Credits: Health and me
What if a single shot could help you lose weight, lower your risk of diabetes, protect your heart, and even cut your chances of developing certain cancers? That’s the promise scientists at Tufts University are chasing with a new experimental drug. Unlike Ozempic or Wegovy, which rely on one or two hormones, this compound combines four. Early research suggests it could deliver weight loss on par with bariatric surgery—without going under the knife and change how we think about treating obesity and the diseases tied to it.
Despite the popularity of drugs like Ozempic and Wegovy, these drugs come with side effects—nausea, bone loss, and weight regain—that limit their long-term potential. Now, researchers at Tufts University believe they may have found a more powerful alternative: a single drug that combines four hormones to tackle obesity and, in turn, the cascade of diseases it fuels, including diabetes, cancer, and cardiovascular disease.
Obesity is not just about excess weight. It is linked to more than 180 conditions ranging from type 2 diabetes and heart disease to certain cancers and liver disorders. According to the World Health Organization, over 650 million adults worldwide live with obesity. In the United States, more than 40% of adults are affected. Treating obesity effectively could ripple across public health, reducing risks of chronic illness and cutting healthcare costs.
That’s what makes the Tufts team’s work so promising. Their “quadruple-action” drug design aims not only to deliver substantial weight loss—up to 30%, on par with bariatric surgery—but also to change how obesity-related conditions are treated at scale.
How Current Weight Loss Drugs Work?
The first wave of modern weight loss drugs works by mimicking hormones released in the gut after a meal. The most prominent of these, GLP-1 (glucagon-like peptide 1), triggers insulin release, lowers blood sugar, and sends signals to the brain that suppress appetite. Ozempic, which is based on GLP-1, has been so effective that the American Diabetes Association now recommends it as a first-line injectable treatment for diabetes.
But GLP-1 drugs have drawbacks. Patients must inject them weekly. Nearly 40% stop after the first month due to intense nausea. Long-term use is associated with bone and muscle loss, and discontinuation often leads to weight regain.
To improve results, drug developers have experimented with combining hormones. Mounjaro (tirzepatide) pairs GLP-1 with GIP (glucose-dependent insulinotropic peptide), which also promotes satiety but reduces nausea. Retatrutide, still in clinical trials, adds glucagon, which boosts calorie burning and suppresses appetite, offset by the glucose-lowering effects of GLP-1 and GIP. This three-hormone chimera has shown weight loss up to 24%—a significant leap from Ozempic’s 6–15%.
What Makes the New 4-in-1 Drug Different?
The Tufts team, led by chemistry professor Krishna Kumar, decided three wasn’t enough. They added peptide YY (PYY), another gut hormone that reduces appetite and slows digestion, but through different pathways than GLP-1 and GIP. PYY may even play a role in fat burning.
Blending PYY with the other three hormones wasn’t simple—it belongs to a completely different structural class. The researchers fused peptide segments end-to-end, creating a new “tetra-functional” compound that engages four distinct receptors at once. The hope is that this design will deliver more consistent results across diverse patients, many of whom respond differently to existing therapies due to genetic or biological variation.
Bariatric surgery remains the most effective intervention for severe obesity, with patients often losing 30% or more of their body weight and keeping it off long term. But surgery is invasive, expensive, and not accessible to everyone. Current drugs fall short of this benchmark. If the new 4-in-1 therapy delivers weight loss on par with surgery, it could transform obesity treatment by offering comparable results without the risks of an operating table.
Graduate researcher Tristan Dinsmore, a co-author on the Tufts study, explained: “We wanted to bring in PYY to complete the weight control quartet. By hitting four receptors at once, we’re aiming for a more balanced, durable effect.”
Tackles Obesity Diabetes, Cancer and Heart Disease
Obesity rarely comes alone. It drives insulin resistance, raising the risk of type 2 diabetes. It fuels inflammation, which is linked to cancer progression. It strains the heart, worsening conditions like heart failure.
At the recent European Society of Cardiology conference in Madrid, large-scale studies revealed that GLP-1-based drugs reduce the risk of hospitalization or premature death among heart patients by as much as 58%. A study published in JAMA further showed that semaglutide (the active ingredient in Ozempic) lowered the risk of heart attack, stroke, or cardiovascular death by 20%, regardless of weight loss achieved.
These drugs are not just cosmetic. They could become a frontline defense against chronic, life-threatening diseases. By adding PYY into the mix, the Tufts candidate drug could amplify these benefits.
Does It Have Any Side Effects?
Side effects remain a stumbling block. For many patients, nausea is so severe that they abandon treatment early. Tufts researchers hope their four-hormone combination will not only boost effectiveness but also improve tolerability. Tirzepatide already demonstrated that blending GLP-1 with GIP reduces nausea; PYY may offer additional relief while protecting muscle and bone mass.
Another challenge is weight regain after stopping treatment. Studies show that weight lost with GLP-1 drugs often creeps back once injections stop. By acting on more pathways simultaneously, the new compound could make weight loss more sustainable, narrowing the gap between drug therapy and surgical intervention.
When Will This New Weight Loss Drug Be Available?
The Tufts research, published in the Journal of the American Chemical Society, is still in preclinical stages. Clinical trials will be the real test, both for safety and for proving whether the quadruple-action therapy can deliver surgery-level weight loss.
If successful, the drug could be a paradigm shift. More than 15 million American adults roughly 4.5% of the population are already using weight loss medications like Ozempic or Wegovy.
Krishna Kumar and his team emphasize that this isn’t just about shedding pounds. “Obesity is linked to over 180 conditions, from diabetes to cancer,” Kumar noted. “What drives us is the idea that we can design a single drug to treat obesity and simultaneously mitigate the risk of developing a long list of health problems plaguing society.”
Mental Health Crisis In 2025 Affects Over 1 Billion People Globally With Unique Anxiety And Depression Patterns

Credits: iStock
Mental health has become one of the most urgent public health issues of the 21st century. Recent statistics from the World Health Organization (WHO) indicate that more than one billion individuals globally live with mental illnesses. Anxiety, depression, and other psychiatric disorders not only cause immense human distress but also carry a massive economic burden, both on individuals and societies as a whole. Where progress has been made in a number of countries to enhance mental health policies and programs, global services remain dramatically underfunded and fragmented, denying access to care for millions.
Mental illnesses are ubiquitous, cutting across all age, gender, and economic strata groups. Anxiety and depression are among the most prevalent disorders, and their effects extend far beyond emotional pain. They are the second global cause of long-term disability, costing money in healthcare, decreasing productivity in the workforce, and lowering quality of life. The fiscal hit is astronomical: depression and anxiety alone have been estimated to cost the international economy $1 trillion each year.
The WHO's recent publications, World Mental Health Today and Mental Health Atlas 2024—set both positive trends and important gaps in mental health services. They are powerful resources to inform national plans and influence the international conversation leading up to the 2025 United Nations High-Level Meeting on noncommunicable diseases, with a focus on mental health and well-being.
Rising Mental Health Concerns Among Young People
Younger populations face particularly intense mental health issues. Gen Z, in particular, is under unprecedented stress from social media, school pressures, and the aftereffects of the COVID-19 pandemic. A 2023 Harvard survey identified that 44% of young adults between ages 18–25 felt like they don't count to others. Further, CDC data indicate that 40% of U.S. high school students indicated they felt sad or hopeless most or all days, and 20% attempted seriously to take their own life. These figures highlight the imperative for accessible and effective mental health care among young people.
Social disruption during the pandemic, from remote learning issues to extended isolation, intensified loneliness and anxiety. Even after lockdowns lifted, many young people still face uncertainty about their futures, academic stress, and the mental health impacts of disrupted childhood or adolescence.
WHO Mental Health Report: One in Every 100 Deaths Worldwide is Caused by Suicide
Suicide continues to be a tragic consequence of mental illness. In 2021 alone, it is estimated that 727,000 individuals across the globe died by suicide, which is a major cause of death among youth. WHO experts point out that although age-specific rates for suicide have fallen worldwide by 35% from 2000 to 2021, efforts are too slow to achieve the United Nations Sustainable Development Goal to cut suicide rates by a third by 2030. The trend indicates only 12% reduction will be realized.
Alarming as it is, almost three-quarters of all suicides are in low- and middle-income countries, where there are limited mental health resources and stigma discourages individuals from going for help. Even in wealthier countries, timely and effective care is not always accessible.
Investment Gap in Mental Health Services
Investment in mental health services globally is not increasing commensurate with growing demand. Median government expenditure on mental health averages only 2% of overall health expenditures—unchanged since 2017. Inequities between nations are glaring: high-income countries can spend as much as $65 per capita on mental health, and low-income nations can spend as little as $0.04. Median numbers of mental health professionals globally stand at only 13 per 100,000 people, and have made low- and middle-income nations critically short.
Access to treatment is most problematic in rural and underserved populations. In the United States, 65% of rural counties have no practicing psychiatrist, and nearly a third have no mental health professionals. Suburban residents, while otherwise better supplied, also experience affordability hurdles, insurance gaps, and cultural stigma, reducing meaningful access to care.
Progress and Persistent Gaps
There has been some progress. In the past two years, most countries have revised their mental health policies, improved emergency preparedness, and incorporated rights-based practices. More than 80% of nations now offer mental health services in emergencies, compared to 39% in 2020. Mental health integrated into primary care is making headway, and telehealth services are increasingly available.
Yet, these developments are insufficient to meet the global burden. Fewer than 10% of countries have fully transitioned to community-based care models, and inpatient care continues to rely heavily on psychiatric hospitals. Many patients experience long-term hospitalization, often involuntarily, highlighting the urgent need for systemic reform.
Why Addressing the Root Causes Is Important?
Mental health is a function of the complex interplay of social, environmental, and biological elements. Social media use, cyberbullying, and the pressure to maintain a "perfect" life on social media can contribute to exacerbating depression and anxiety. Economic insecurity, discrimination, trauma, and the residual effect of global crises such as the pandemic further add to the burden. Resolution of these foundational issues demands intersectoral collaboration—healthcare, education, social services, and policy.
Simple Tips for Improving Your Mental Health Everyday
Although reform on a wide scale is called for, people can also take actions to augment their mental health:
Stay Connected: Regular contact with others reduces loneliness.
Prioritize Physical Health: Exercise, healthy nutrition, and sleep contribute heavily to mood and cognitive performance.
Limit Digital Overload: Cut back on social media time, especially doomscrolling or comparing yourself to idealized models.
Practice Mindfulness: Meditation, journaling, or breathing exercises can reduce stress and enhance emotional resilience.
Get Professional Assistance: Therapy, counseling, or support groups provide direction and management techniques.
Foster Open Discussions: Open discussion of feelings within families, schools, or the workplace decreases stigma and promotes early intervention.
Crisis Hotlines: Familiarize yourself with local or national hotlines. For example, Kosovo provides Lifeline at 0800 12345 between the hours of 10:00 AM to 2:00 AM every day for crisis intervention.
The WHO underlines that mental health services should be addressed as a human right. Radical change to mental health services requires fair financing, legal changes to ensure human rights, and continued investment in the development of the workforce. Community-based, person-focused care models are essential to increase access and enhance outcomes. Multilevel collaboration between governments, NGOs, and international health agencies is required to address the breadth and depth of the crisis.
The current statistics present a grim picture: mental illness disorders are growing more quickly than world population growth, suicide is a leading cause of death among young people, and treatment access is starkly uneven. Unless drastic action is taken, the economic, social, and human toll will keep piling up.
Mental illness is not only a matter of health; it is a societal and economic problem that needs to be addressed immediately. Over one billion individuals are impacted globally, and younger generations disproportionately so. Progress has been made in policy, integration, and emergency response, yet never before has systemic reform and investment been as urgent a need. There is a role for every government, community, and individual in opening up access, decreasing stigma, and placing mental health as a top global public health priority.
New AI Stethoscope Can Detect Serious Heart Conditions In Just 15 Seconds
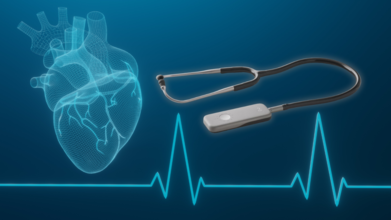
Credits: Imperial College Of London
For over two hundred years, the stethoscope has been an symbol of medical care, a humble but indispensable device permitting physicians to hear the internal beat of the human body. Since the invention in 1816, the model has not changed a great deal. Now, scientists at Imperial College London and Imperial College Healthcare NHS Trust have unveiled a high-tech upgrade: an artificial intelligence (AI)-driven stethoscope that can diagnose heart failure, heart valve disease, and irregular heart rhythms in only 15 seconds.
The AI stethoscope is a breakthrough in cardiac treatment. In contrast to conventional stethoscopes that are dependent on the skilled ear of a physician, this one picks up subtle changes in heartbeat and blood flow that cannot be detected by humans. Simultaneously, it performs a quick electrocardiogram (ECG) to capture the electrical activity of the heart. This simultaneous process enables detection of three significant cardiovascular diseases almost instantly, which could revolutionize the way heart disease is diagnosed in primary care.
These conditions must be identified early. Atrial fibrillation, valve dysfunction, and heart failure can go quietly, only being detected when the patients are seriously ill. Dr. Patrik Bächtiger, from Imperial College London's National Heart and Lung Institute, explains the implications: "The stethoscope design has not changed in 200 years—until now. A 15-second diagnosis can now provide actionable insight that was previously the result of multiple tests."
The technology was piloted in the Tricorder trial, a randomized controlled study of about 12,725 patients across 200 general practice surgeries throughout the UK. Patients had symptoms like breathlessness, tiredness, or swelling of the legs, all typical signs of heart failure. Researchers made comparisons between outcomes for patients seen with the AI stethoscope and those seen through usual care without the device.
The outcomes were impressive. Patients evaluated using the AI stethoscope were more than twice as likely to be diagnosed with heart failure in the next year. Atrial fibrillation, an irregular heartbeat that greatly ups stroke risk, was detected more than three times as often, and diagnosis of heart valve disease almost doubled. These findings suggest that AI-aided auscultation can significantly boost the early identification of potentially fatal cardiovascular diseases.
How the AI Stethoscope Works?
The gadget, made by California-based firm Eko Health, is about the size of a playing card. Once positioned against a patient's chest, its microphone picks up the sound of blood flow as a quick ECG captures electrical activity. It sends the information securely to cloud-based servers, where the AI algorithms trained on tens of thousands of recordings from patients process the signals. Results are reported to a clinician's phone within minutes, indicating if the patient is at risk of one of the three cardiovascular conditions.
"Having the capability to integrate sound analysis with ECG in real-time is unparalleled," states Dr. Mihir Kelshiker from Imperial College. "This method enables GPs to spot issues prior to patients coming into emergency care, which could save lives and help curb healthcare expenditures."
Advantages and Considerations
More than speed and accuracy, the AI stethoscope provides a scalable platform for community-based care. NIHR's scientific director of innovation, Prof. Mike Lewis, comments: "By getting diagnosis innovation into GP clinics, we give local clinicians the power to detect serious conditions earlier, tackling some of society's biggest health issues."
That said, there are some limitations to the technology. The researchers warn that the device needs to be applied in symptomatic patients instead of being used for screening healthy persons in routine. False positives have a risk of occurring in which the patient will be mistakenly identified as being at risk, the implication being that the clinical context is crucial in interpretation.
How Could AI Stethoscope Transform Cardiovascular Health?
For many years, cardiovascular disease has been a top cause of death globally, frequently complicated by delayed diagnosis. The AI stethoscope is a breakthrough, allowing earlier intervention by clinicians, more personalized treatments, and better long-term outcomes. Dr. Sonya Babu-Narayan, clinical director at the British Heart Foundation, highlights the potential benefit: "Earlier diagnosis means people can receive life-saving treatments earlier, enhancing quality of life and survival."
The equipment also vows to simplify care pathways. By quickly flagging up high-risk individuals in primary care, it can cut back on the necessity for repeated follow-up tests and specialist referrals, freeing up hospital capacity and enhancing patient satisfaction.
After the trial, the intention is to roll out the AI stethoscope to GP practices across Wales, South London, and Sussex. Broader uptake is expected to prove its efficacy with a wide range of patient populations and healthcare environments. Further development will add more sophisticated cardiac diagnostics to the device and continue to improve AI algorithms to make it more accurate.
As the technology advances, the stethoscope AI highlights a larger trend in medicine: the coming together of artificial intelligence and old clinical devices. It shows how ancient devices that date back centuries can be rediscovered for the 21st century, closing the distance between early detection and timely intervention.
From its modest beginnings in 1816 to the AI-assisted stethoscope today, the stethoscope remains at the heart of medicine, now poised to revolutionize cardiovascular diagnostics. By allowing for rapid, precise identification of heart failure, atrial fibrillation, and valve disease, the AI stethoscope might save thousands of lives, alleviate the load on healthcare systems, and redefine the way heart disease is treated worldwide. For clinicians and patients alike, it is a step forward in the quest for quicker, smarter, and more accurate cardiac care.
© 2024 Bennett, Coleman & Company Limited

