- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
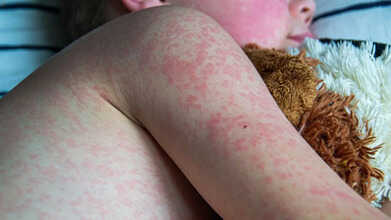
As U.S. Measles Cases Surge, Could The Country Lose Its Elimination Status?
Credits: Canva
Measles has held “eliminated” status in the United States since 2000, a designation that means there had been no year-long, uncontrolled spread of the virus within the country for decades. But that milestone is now in jeopardy.
The infection is spreading again, and the Centers for Disease Control and Prevention (CDC) has reported 1,596 confirmed cases this year, the highest annual total in over thirty years. The actual number could be higher, says Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Also Read: Texas Sues Tylenol Makers After Trump Links Painkiller To Autism — All You Need to Know
Measles Case Surge In The US
Since August 2025, more than 100 people have been infected in the Utah–Arizona border region, forming the second-largest measles cluster in the U.S. this year. Most patients were unvaccinated, and the virus has now moved beyond small religious circles into the general population. This outbreak followed the earlier “Southwest wave,” which infected over 880 people in Texas, New Mexico, and Oklahoma, sending national measles numbers to a 34-year record high.
Experts warn that the current pattern resembles the major outbreaks seen in the early 1990s, before widespread vaccination programs curbed transmission. The largest outbreak occurred in West Texas, where 99 people were hospitalized and two unvaccinated school-age children died — the first measles deaths in the country since 2015. The Texas Department of State Health Services declared that outbreak over in mid-August. New Mexico has also reported a measles-related death, according to CNN. In response, the CDC has sent emergency teams to Utah, Arizona, Minnesota, and South Carolina to help limit the spread.
Also Read: The 'BEFAST' Trick Could Help You Detect Your Stroke Before It Happens, According To Doctor
What Is Causing Measles to Increase in the U.S.?
In Mohave County, Arizona, vaccination coverage among kindergartners dropped from 91% in 2019–20 to 78% in 2024–25, with a similar decline noted in southwest Utah.
Public health experts stress that at least 95% vaccination coverage is necessary to prevent the virus from spreading. Both Utah and Arizona allow parents to exempt children from school vaccine mandates on personal or religious grounds, leaving pockets of unprotected populations.
According to The Times of India, vaccine hesitancy has deepened since the Covid-19 pandemic, fueled by political polarization and a decline in public trust toward health authorities. Notably, most of the affected towns voted heavily for Donald Trump in the 2024 election, underscoring how politics and misinformation continue to shape vaccination decisions.
Will the U.S. Lose Its Measles Elimination Status?
In November, the Pan American Health Organization (PAHO), the regional arm of the World Health Organization (WHO) will hold its annual Measles and Rubella Elimination Regional Monitoring and Re-Verification Commission meeting to review each country’s elimination status.
The WHO defines a loss of elimination status as continuous transmission lasting longer than 12 months. While there are no direct penalties, re-establishing measles as an endemic disease would mark a major public health setback, leading to avoidable deaths and straining local healthcare systems.
Countries that lose their elimination status are required to submit a corrective action plan, typically involving intensive vaccination efforts, stronger surveillance, and faster outbreak responses.
Although President Donald Trump signed an executive order in January to withdraw the U.S. from the WHO, PAHO officials said they still expect representatives from the CDC and the National Sustainability Commission to attend next month’s meeting.
In August, local health authorities declared the end of the West Texas outbreak, which had caused 762 infections over seven months, according to state data. Neighboring New Mexico also reported 100 linked cases and one adult death, bringing the total fatalities from the outbreak to three which were two children in Texas and one adult in New Mexico, all unvaccinated.
Despite these containment efforts, new clusters continue to appear in Utah, Arizona, and South Carolina, and investigators are examining whether these are connected to the Texas outbreak. If transmission persists into January, the United States could officially lose the measles elimination status it earned in 2000.
The CDC calls the elimination of measles one of the country’s most significant public health achievements, made possible through a highly effective vaccination program and improved control efforts across the Americas. However, MMR vaccine coverage in both the U.S. and Canada has been steadily falling, with sharper declines noted after the Covid-19 pandemic.
Wet Wipes Warning: Patient Dies Using An Infected, Non-Sterile Wipe

Credits: Canva
Wet Wipes Waring: 59 people have fallen ill, with one dead, after using non-alcoholic, non-sterile, infected wet wipes in UK. The UK Health Security Agency (UKHSA) and Medicines and Healthcare products Regulatory Agency (MHRA) issued a warning, and said that there is still "an ongoing risk of infection associated with their use". The bacteria that is causing this infection is burkholderia stabilis or B. stabilis.
Wet Wipes Warning: Which Ones Not To Use

The UKHSA also in its warning included names of four different wet wipes and asked people to avoid using them.
- ValueAid Alcohol Free Cleaning Wipes
- Stereoplast Sterowipe Alcohol Free Cleansing Wipes
- Reliwipe Alcohol Free Cleansing Wipes
- Microsafe Moist Wipe Alcohol Free
Wet Wipes Warning: What Actually Happened?
The Pharmaceutical Journal noted that the UKHSA and MHRA published a joint statement that warned people that they should not use non-sterile, non-alcoholic wipes in their homes and first-aid kits.
After an outbreak investigation conducted by MHRA in 2025, four products were identified to be contaminated with Burkholderia stabilis. “There have been 59 confirmed cases of Burkholderia stabilis associated with some non-sterile alcohol-free wipe products — identified in an outbreak in the United Kingdom from January 2018 to 3 February 2026,” the statement said.
“A small number of cases continue to be detected. These have included some serious infections which have required hospital treatment and one death has been attributed to Burkholderia stabilis infection.”
UKHSA and MHRA is telling people to look out for wipes marked 'sterile' to be used on wounds or broken skin.
Wet Wipes Warning: What Is Burkholderia Stabilis?
UKHSA on its official website notes: 'Burkholderia are a type of bacteria found naturally in the environment, including in soil and water. Burkholderia stabilis is one species within this group. While many people never encounter problems with this type of bacteria, it can cause serious infections in certain circumstances, particularly among vulnerable individuals.'
Read: Does Bigger Penis Help You Ski Better? Why Olympians Are Injecting Hyaluronic Acid - Explained
Wet Wipes Warning: Who Is At More Risk Of B. Stabilis Infection?
- Those with weakened immune systems – including people undergoing chemotherapy, organ transplant recipients, or those with conditions that affect immune function
- Individuals with other risk factors such as cystic fibrosis
- Patients at home with intravenous lines
Wet Wipes Warning: How Can Someone Get Infected By B. Stabilis?
The UKHSA notes: "The risk of acquiring infection is generally very low. Infections can occur through contact with contaminated products on broken or damaged skin, or through introduction of bacteria through medical devices such as intravenous lines."
Wet Wipes Warning: What Are The Common Symptoms Of B. Stabilis Infection?
- Symptoms of a wound infection can include redness, swelling, increased pain, warmth around a wound or or break in skin, and pus or other drainage from the wound / break in skin
- Symptoms of infection involving an intravenous line can include signs such as redness, swelling, or pain around the insertion site and / or fever and chills
- In more serious cases, symptoms associated with bloodstream infection (sepsis).
Delhi Kidnappings Were False Fear Mongering, Officials Confirm

Days after it emerged that over 800 people had gone missing in Delhi during the first 27 days of 2026 and officials requested people to remain calm, Delhi Police has revealed this is false and such claims are being "pushed through paid promotion".
In a statement released on X today morning: "After following a few leads, we discovered that the hype around the surge in missing girls in Delhi is being pushed through paid promotion. Creating panic for monetary gains won't be tolerated, and we'll take strict action against such individuals."
Authorities across the capital claim that dispensing false information about life-altering information such as kidnappings and death through paid promotion can cause as well as amplify fear among the general public, which can massively ruin mental health and cause long-term damage to overall wellbeing.
Dr Kunal Bahrani, Chairman and Group Director, Neurology at Yatharth Hospitals, explained to Healthandme: "Incidents like this highlight how quickly fear can spread in today’s hyperconnected world and how deeply false news can impact mental wellbeing. When people are exposed to alarming information, especially involving safety and crime, the brain’s threat system switches on almost instantly. Stress hormones such as cortisol surge, increasing anxiety, restlessness, sleep disturbances and even physical symptoms like headaches or heart palpitations.
"Repeated exposure to frightening but unverified news can also condition the brain to remain in a constant state of alert, making individuals more irritable, emotionally overwhelmed and prone to panic. For children, elderly people and those already struggling with anxiety or depression, the psychological impact can be far more intense and long-lasting."
However, while many wonder how this form of promotion came into being, Reddit users are already pointing towards Rani Mukerji's Mardaani-3, a movie based on the kidnapping of minor girls as the source for this chaos.
How Does Fear Mongering Ruin Mental Wellbeing?
Fear-mongering severely impacts mental health by triggering chronic stress, anxiety, and depression, often overloading the brain’s threat response system (amygdala) and leading to long-term damage.
Over time, constant exposure to exaggerated, alarming narratives fuels irrational fears and cognitive distortions such as catastrophizing. This constant state of fear can also lead to avoidance behaviors, hopelessness, and physical symptoms like headaches, poor sleep and a weakened immune system.
Dr Neetu Tiwari, MD Psychiatry Senior Resident, NIIMS Medical College & Hospital also told Healthandme: "When things like this happen around us, it creates a sense of panic and fear amongst parents and children. Our brain usually reacts as if the danger is real. The body then becomes alert, our heart beats faster than it should, and our mind stays at tense. Even if the news is proven to be false, this fear that has set in often takes time to settle down.
"Now this in turn causes a lot of trouble. It makes people feel unsafe, so people avoid going out, they worry more about loved ones, or may also keep checking their phones repeatedly for updates. This behavior can lead to anxiety, panic, disturbed sleep, and constant overthinking. Even when the news is fake, fear travels very quickly. And once fear enters our mind, calming down takes time.
"This is why sharing unverified information can unknowingly harm others especially children as it can lead them in feeling scared even to go to school, or sleep alone, or even step outside. In fact in some children this may even cause nightmares or these children become unusually clingy to parents or guardians which further leads to problem in social development and confidence.
Dr. Rajiv Mehta, senior consultant psychologist, Sir Gangaram Hospital added: "The society at large is very gullible and anxiety prone, as we have seen the rise in mental illnesses. The people who are already anxious will get more anxious, the people who are not anxious will get anxious and that will increase the mental instability in the persons.
"And what they and parents are going to do is take a lot of precautions towards their young kids by not allowing them to go outside, to play with the children and go out of society buildings. They will be very fearful and when children will be home bound, then they will spend more time on screens or will have altercations with their parents.
"People should not resort to such kind of things only for the sake of their own materialistic monetary benefit. That is highly condemnable if it has occurred because of these kind of reasons that movie is going to release and they are sensationalizing these things."
Are The Delhi Kidnappings False?
Data obtained from Press Trust of India suggests that a total of 807 people went missing between January 1 and 15, with an average of 54 people going missing every day. Of these, 509 were women and girls, and 298 were men. Among the total reported missing, 191 were minors and 616 were adults.
In an official statement, the police said that, while the data was recorded, January 2026 saw a "decline in the number of missing persons reports when compared with the corresponding period of previous years."
Officials had previously further clarified that no organized gang or criminal network has been found involved in cases of missing or abducted children in Delhi so far.
"Recent public discourse has raised concerns about the welfare of children in Delhi. We appeal to the citizens not to fall prey to the rumours about the spurt in the cases of missing children.
While denying such claims, we also warn rumour mongers of strict legal action for creating unnecessary panic and fear by misrepresenting data.
The safety of every child is of paramount importance to Delhi Police.
"Delhi Police is committed to serve 24x7 and making all out efforts to trace all missing persons and reunite them with distraught family members, at the earliest," they said in a statement released yesterday.
Cheaper Copy Of Wegovy Pill By Hims & Hers Now Available For 49, Novo Nordisk Says It Will Take Legal Actions

Credits: iStock
Cheaper copy of Wegovy by Hims & Hers for $49 has drawn tensions, with Novo Nordisk now approaching to take legal actions. The pill by Novo Nordisk is sold for $149. In a statement, Novo said, "The action by Hims & Hers is illegal mass compounding that poses a significant risk to patient safety. Novo Nordisk confirmed that the company will take legal and regulatory actions to protect patients.
“Novo Nordisk will take legal and regulatory action to protect patients, our intellectual property and the integrity of the US gold-standard drug approval framework. This is another example of Hims & Hers’ historic behaviour of duping the American public with knock-off GLP-1 products, and the FDA has previously warned them about their deceptive advertising of GLP-1 knock-offs,” the statement said.
Read: Wegovy Pills Now Available At Your Pharmacies, Here's What To Know About Its Usage
Cheaper Copy Of Wegovy Pill: What Unveiled Afterwards?
After the cheaper copy of the pill was launched by Hims & Hers, shares of Novo Nordisk and rival Eli Lilly fell by 7%. Whereas, stock for Hims & Hers initially spiked after the announcement, but pared gains after Novo said that it would fight the rollout.
Hims & Hers was previously offering compounded semaglutide, the active ingredient in Novo Nordisk's popular weight loss drug Ozempic and Wegovy, in injectable format. The company has now extended to offer the oral version.
Cheaper Copy Of Wegovy Pill: How Much Does This Cost?
Hims & Hers said that the Wegovy copy pill cost $49 for the first month and $99 with a 5-month plan. While semaglutide's patent is protected in the US until 2032, however, Hims & Hers claim that the copies are 'personalized' and therefore legal.
“This compounded product uses a different formulation and delivery system than FDA-approved oral semaglutide,” Hims & Hers said. “This once-a-day pill has the same active ingredient as Wegovy and empowers providers to tailor treatment plans specifically for those who prefer to avoid needles or need smaller doses to help to balance side-effects,” it said.
Cheaper Copy of Wegovy Pill: Novo Nordisk Rejects Hims & Hers Claims
Novo Nordisk however, highlighted that it manufactures Wegovy pill by using SNAC technology. This technology helps in absorption when administered orally. Therefore, it is not clear how Hims & Hers copy formula could match that level of absorption.
Last year, Novo Nordisk and Hims & Hers partnered to offer weight loss jabs at a discounted price to telehealth company's customer. However, Novo Nordisk ended the collaboration just two months later, stating that Hims & Hers used 'deceptive' marketing that put patient safety at risk
Cheaper Copy of Wegovy Pill: Does Eli Lilly Have A Weight Loss Pill Yet?
While rival Eli Lilly does not have the weight loss pill yet, it is expected to launch orforglipron in the first half of this year. The pill is currently pending Food and Drug Administration approval.
Read: Eli Lilly Sends Weight-Loss Pill For Approval: Is Oral GLP-1 As Effective As The Injections?
“With the ... current legal backdrop, there is no reason why HIMS shouldn’t evaluate these launches for every subsequent weight loss product as the market continues to evolve,” Leerink analyst Michael Cherny said in a note to clients.
© 2024 Bennett, Coleman & Company Limited

