- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Can AI Predict Your Risk Of Cancer? FDA Approved A New Tool That Spots Disease 5-Year Before It Appears
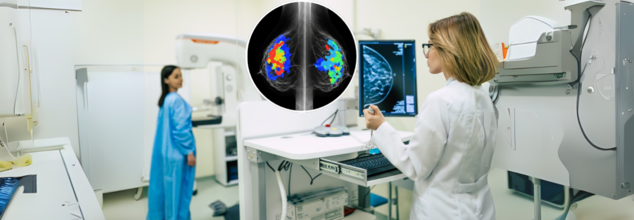
The U.S. Food and Drug Administration (FDA) has authorized the first-ever artificial intelligence (AI) tool designed to predict a woman’s five-year risk of breast cancer using a routine screening mammogram. The announcement, made on May 30, 2025by the tool’s developer, Clairity, marks a pivotal step in how breast cancer is detected and prevented—and it may just redefine the standard of care in women’s health.
This isn’t just another AI detection tool. Clairity’s platform, known as CLAIRITY BREAST, is not focused on spotting existing tumors, but rather on forecasting who may develop breast cancer years in advance. It shifts the paradigm from early detection to early risk prediction, potentially giving clinicians a critical head start in identifying high-risk individuals—before cancer ever forms.
This innovation comes from the vision of Dr. Connie Lehman, a radiologist and professor at Harvard Medical School, and the former chief of breast imaging at Massachusetts General Hospital. With decades of experience detecting cancers on mammograms, Lehman realized a key gap in screening: the lack of modern, image-based risk assessment tools.
“Our imaging technology was advancing fast, but our methods to identify women at high risk weren’t keeping up,” said Lehman at the 2025 American Society of Clinical Oncology (ASCO) Meeting, where the FDA authorization was announced.
While most existing risk models consider age, genetics, and family history, they often miss women who don’t fit those boxes. In fact, 85% of women diagnosed with breast cancer have no family history, and 50% have no identifiable risk factors at all. This makes Clairity’s approach uniquely valuable.
How The Breast Cancer Detection AI Tool Works?
CLAIRITY BREAST leverages deep learning and computer vision algorithms trained on millions of mammogram images, each linked to five-year follow-up data. By analyzing the subtle imaging features in breast tissue—often imperceptible to the human eye—the system generates a validated five-year risk score for each patient.
That score is integrated seamlessly into existing clinical workflows, providing radiologists and oncologists with a powerful new layer of information for decision-making.
“Now we can move even further upstream,” said Lehman. “Not just detecting cancer early—but predicting it, personalizing care, and even preventing it.”
Traditional screening strategies are reactive, catching cancer once it’s already present. But with Clairity, the approach becomes proactive, especially for women who may otherwise fall through the cracks of conventional risk models.
“This FDA authorization is a turning point,” said Dr. Larry Norton, founding scientific director at the Breast Cancer Research Foundation. “Most existing risk models miss the very women who go on to develop breast cancer. Clairity changes that.”
Younger women in particular stand to benefit. While breast cancer incidence is still highest in women over 50, cases among younger women are rising, yet they’re often not recommended for routine screening unless they have known risk factors. Clairity’s AI-powered insights can help clinicians tailor screening plans and intervene earlier for women who previously would have gone unflagged.
Dr. Lehman describes breast cancer not as a binary—either present or not—but as a continuum, with progression that begins long before a tumor is visible.
“There’s a point even before ductal carcinoma in situ (DCIS), when cancer hasn’t developed, but the risk is there,” she explained. “That’s where CLAIRITY can intervene—well before invasive cancer begins.”
The goal, according to Lehman, isn’t just to detect breast cancer earlier, but to predict and prevent it, in the same way that doctors routinely assess cardiovascular risk based on blood pressure, cholesterol, or BMI.
Until now, breast cancer lacked such a dynamic, image-based risk tool. That’s what makes Clairity’s authorization so significant.
AI’s Expanding Role in Radiology
AI has been used in radiology for decades, first to flag microcalcifications in mammograms in the early '90s. But back then, AI systems were limited to rule-based logic and needed exhaustive human guidance.
Today’s AI, especially in platforms like Clairity, goes far beyond what human vision can perceive. It identifies patterns in breast tissue texture and density at the pixel level, learning from vast image sets without needing explicit human-labeled inputs.
Lehman compared it to how machines learn to distinguish cats from dogs—by exposure to thousands of examples rather than being told what features to look for. In the case of breast cancer, the stakes are obviously much higher.
“This technology allows us to see what we couldn’t see before, and that opens doors to dynamic risk assessment,” Lehman said. “Eventually, we may even use these tools to monitor how interventions like medication, weight loss, or hormone therapy change a woman’s risk over time.”
Backed by Santé Ventures and ACE Global Venture, Clairity was founded in 2020 with a clear goal: to bring sophisticated AI tools to routine mammography. Now, with FDA de novo authorization, it’s set to hit the market by late 2025.
The company estimates the global market for breast cancer prediction tools at $63 billion, suggesting broad clinical interest and commercial potential.
As imaging centers, hospitals, and oncologists look to integrate risk prediction into daily care, Clairity's success will depend on adoption, clinician trust, and real-world outcomes. But with this regulatory milestone, a major hurdle has been cleared.
The FDA’s green light for CLAIRITY BREAST doesn’t just add a new tool to the cancer detection toolbox—it fundamentally changes the playing field. By enabling risk prediction from a single mammogram, it turns routine screening into a forward-looking, personalized roadmap for prevention.
Over 8 Million U.S. Teens Now Prediabetic, CDC Warns; Can This Health Crisis Be Reversed With Lifestyle Tweaks?

Credits: Freepik
The CDC has just delivered a reality check revealing over 8.4 million American teens aged 12 to 17—roughly one in three—are prediabetic. That’s 32.7% of U.S. adolescents showing early signs of blood sugar trouble that could spiral into full-blown type 2 diabetes. And this isn’t just about elevated glucose levels. This is a window into a much larger crisis: preventable chronic illness silently growing among kids who haven’t even finished high school.
“This is a wake-up call,” says Dr. Christopher Holliday, the CDC’s Director for Diabetes Translation, pointing to the massive and preventable health burden the country now faces. The warning is clear—teen health is declining, and unless there’s a nationwide shift in how we approach diet, movement, stress, and sleep, things are going to get worse before they get better.
Prediabetes means your blood sugar levels are elevated but not high enough for a diagnosis of type 2 diabetes. It’s like standing at the edge of a cliff—you’re not falling yet, but you’re dangerously close.
During puberty, a teen’s body undergoes hormonal shifts that can naturally interfere with how well insulin works. According to Yale Medicine, this makes adolescence a critical window for diabetes risk to take root. Without intervention, prediabetes can evolve into type 2 diabetes, paving the way for serious health complications including kidney damage, stroke, and cardiovascular disease.
Type 2 diabetes itself is a long-term condition where the body struggles to use insulin properly. Over time, blood sugar builds up in the bloodstream and starts damaging tissues and organs. The progression is slow and mostly silent. Many don’t even know they’re prediabetic until symptoms become too obvious to ignore.
The CDC's 2023 data isn’t the first red flag. A 2020 study published in JAMA Pediatrics revealed that the prediabetes rate in teens more than doubled between 1999 and 2018—from 12% to 28%. The latest CDC estimate of 32.7% suggests that the trend hasn’t just continued—it’s accelerating.
What’s even more sobering is how unevenly this crisis affects different groups. Teens living in poverty are significantly more likely to be prediabetic, tied to issues like food insecurity, poor access to healthcare, and systemic inequality. Research from the University of Pittsburgh connects prediabetes risk with lack of insurance and household incomes below 130% of the federal poverty level.
And there’s a racial disparity too: African American, Hispanic/Latino, American Indian, Alaska Native, Pacific Islander, and some Asian American communities carry a disproportionately high burden of prediabetes and type 2 diabetes.
Is This Collision of Lifestyle and Access?
There’s no single culprit. But a combination of poor nutrition, sedentary habits, limited access to safe outdoor spaces, ultra-processed food marketing, and rising stress levels among adolescents are all playing a role.
Let’s be honest—the modern American teen lifestyle is working against metabolic health. Fast food is cheap and available everywhere. Physical education has been reduced or eliminated in many schools. Screen time has skyrocketed, especially since the pandemic. Many families, particularly those struggling financially, don’t have the luxury of prioritizing healthy eating or gym memberships.
Add to that a healthcare system that often fails to screen for prediabetes in young people, and you’ve got a perfect storm.
Can Lifestyle Tweaks Actually Reverse Prediabetes?
Yes—and this is where the good news comes in. According to the American Diabetes Association, prediabetes can be reversed or delayed through sustainable, everyday choices. Dr. Holliday emphasizes that “simple life changes—like healthy eating and staying active—can make a big difference.” Here’s what makes the biggest impact:
- Teens should be active at least three times a week—but ideally more. This doesn’t mean hours at the gym. Walking, biking, dancing, or playing a sport counts.
- Meals should center around whole foods—vegetables, fruits, whole grains—while cutting back on sugary beverages, red meats, and refined carbs.
- Even a 5–7% reduction in body weight in overweight individuals has been shown to significantly lower diabetes risk.
- Chronic stress raises cortisol, which can mess with blood sugar. Meanwhile, lack of sleep can interfere with insulin function. Most teens need 8–10 hours per night.
These aren’t drastic changes. In fact, experts say even modest shifts in habits can dramatically reduce risk.
Is This A Problem of Awareness and Urgency?
One of the most troubling aspects of prediabetes is how few people know they have it. Among American adults, more than 80% of those with prediabetes are unaware. And in teens, the lack of screening and routine checkups makes it even easier for warning signs to go unnoticed.
The key now is early intervention. Pediatricians, schools, and families need to be having these conversations. Blood sugar testing should be routine for at-risk teens. And public health efforts must prioritize communities most affected—those dealing with food deserts, high poverty rates, and systemic barriers to care.
What Needs to Change at a National Healthcare Level?
This isn’t just a family-level issue. It’s a national health crisis that demands systemic action. Here’s what needs to shift:
Healthcare policy: Expand routine screenings for teens, especially in underserved populations.
Food access programs: Subsidize fresh produce in low-income areas and restrict junk food marketing to kids.
School reforms: Reinstate physical education, revamp cafeteria offerings, and make mental health counseling widely available.
Community initiatives: Fund safe recreational spaces, after-school sports, and education campaigns targeting families and caregivers.
The fact that over 8 million U.S. teens are already prediabetic should stop us in our tracks but this isn’t a lost cause. Prediabetes doesn’t have to become diabetes. The road ahead doesn’t require miracles—just smarter choices, better systems, and the will to prioritize adolescent health. If the U.S. can treat this data as the urgent warning it is, we can flip the script on youth diabetes before it's too late.
Could A ‘Harmless’ Virus Be Behind Parkinson’s? New Study Raises Big Questions

Credits: Canva
A virus long thought to be harmless might now play a key role in unraveling the mystery of Parkinson's disease. Human Pegivirus (HPgV), a virus that is normally found in blood and which was long believed to be harmless, has been found in patients diagnosed with Parkinson's disease in their brains. The research published on July 8 in JCI Insight has the potential to change scientists' understanding of the origin of the neurodegenerative disorder that strikes over 1 million Americans.
Guided by Dr. Igor Koralnik, Northwestern Medicine chief of neuroinfectious diseases and global neurology in Chicago, the study detected HPgV in 50% of Parkinson's patients' autopsied brains—but none in the control group. "We were surprised to detect it in the brains of Parkinson's patients at such high frequency and not in controls," Koralnik said.
HPgV, a member of the same virus family as hepatitis C, is usually symptomless and generally overlooked in clinical practice. Nevertheless, this new finding indicates that HPgV is possibly not as harmless as previously thought. The virus was found not only in the brains of infected patients but also in their spinal fluid—a sign that it has possibly infiltrated the central nervous system.
Perhaps more fascinating is how individuals' immune systems respond to HPgV. These responses, says Koralnik, were highly variable and influenced by genetic considerations. Specifically, individuals carrying the Parkinson's-linked LRRK2 gene mutation exhibited unique immune responses, implying a possible interaction between the virus and the body's genetic topology.
Parkinson's disease is mostly an idiopathic condition—i.e., most instances occur without a recognized genetic etiology. This has prompted researchers for many years to pursue environmental etiologies, such as toxin exposure and head injury. The potential viral link adds another twist to that theory. If HPgV is found to be causal, it would be one of the first known environmental factors directly implicated in Parkinson's disease development.
This implies it might be an environmental factor that acts on the body differently than we previously understood," said Koralnik. "It might affect how Parkinson's arises, particularly in individuals with specific genetically derived backgrounds."
Why HPgV Could Matter More Than We Thought?
The researchers autopsied the brains of 10 Parkinson's patients and matched them with 14 brains of healthy individuals. HPgV was found in half of the Parkinson's brains but none of the healthy brains. Further, scientists detected evidence of more damage to the brain in the virus-positive patients, which further solidified the connection.
They next examined blood samples from more than 1,000 people from the Parkinson's Progression Markers Initiative, a large, ongoing study supported in part by the Michael J. Fox Foundation. Just 1% of those with detectable HPgV in their blood, which suggests that the virus can evade detection or pass into the brain without being detected using routine blood tests.
Parkinson's disease is marked by the death or damage of dopamine-producing neurons of the basal ganglia, which is a region of the brain that controls movement. When dopamine levels decline, symptoms such as trembling, stiffness, and poor coordination start to develop. But the disease also produces non-motor symptoms resulting from damage in other locations—such as fatigue, gastrointestinal problems, and blood pressure abnormalities.
How Parkinson's Usually Progresses in the Brain?
A characteristic of Parkinson's is the presence of Lewy bodies, aggregations of misfolded alpha-synuclein protein within brain cells. These deposits are thought to lead to cell death and loss of cognitive function.
The new study opens up the possibility that HPgV either directly injures neurons or provokes immune reactions that make alpha-synuclein aggregation worse. Both possibilities offer new leads for investigation, diagnosis, and ultimately treatment.
As Parkinson's advances, most patients develop cognitive symptoms ranging from mild forgetfulness and difficulty concentrating to frank Parkinson's dementia. This dementia is closely associated with Lewy body accumulation and progresses over time.
Stress, depression, and some drugs make cognitive symptoms more severe, so early detection and management are important. If HPgV is found to be affecting these changes in cognition, it would reveal the possibility of preventive interventions targeting viral suppression or immune modulation.
This research is just the start. Koralnik and his colleagues are now working on two main questions: how frequently does HPgV invade the brain, and how does its invasion relate to the severity of the disease? They're also exploring whether there are other viruses with such effects, particularly in people with genetic mutations such as LRRK2.
"We also want to know how genes and viruses communicate with each other; information that may uncover why Parkinson's starts and may inform the next generation of therapies," Koralnik said.
Future research might investigate whether antiviral treatments or vaccines could slow the development of Parkinson's in virus-positive individuals. Another option is the use of HPgV as an earlier disease biomarker or disease tracking.
This study highlights an overarching trend in medicine today: the reevaluation of microbes previously known to be innocuous. From the bacteria in our guts to the latent viruses in our systems, we are finding that these innocuous-looking commuters on our body highways have significant long-term impacts on health.
For families and patients with Parkinson's disease, this study provides a glimmer of hope—if not for a quick fix, then for better understanding and more individualized means of care.
As with so many scientific advances, the discoveries raise as many questions as they resolve but for a disease as multifaceted and life-changing as Parkinson's, even a step in the direction of shedding light on its causes is a leap of giant proportions toward future treatments.
Colon Cancer Could Kill 53,000 Americans In 2025, Experts Say The American Diet Is To Blame
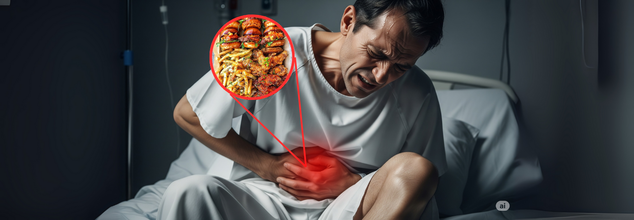
Credits: Health and me
Colon cancer is no longer an old person's disease. In a recent turn of events, incidence rates for colorectal and other GI cancers are increasing steadily in Americans under the age of 50, which is concerning scientists, physicians, and public health experts worldwide.
Based on the American Cancer Society, in 2025 about 52,900 Americans are predicted to be killed by colorectal cancer, with a disproportionate number of deaths falling among young adults—some in their 20s and 30s. The question that experts are desperately seeking to answer is: Why now?
At the center of the crisis is a hypothesis that's gaining momentum: the American diet and increasing levels of obesity are fueling early-onset colon cancer.
Colorectal cancer is the second leading cause of cancer death in the U.S., and a study published in the journal BJS by Oxford University Press indicates this increase is strongly associated with obesity-related circumstances like chronic inflammation, raised insulin, and non-alcoholic fatty liver disease.
A 2019 study identified that women who were defined as obese had almost twice the risk of early-onset colorectal cancer compared to their non-obese peers. With a projection showing that about half of all U.S. adults will be obese by 2030, the association is increasingly difficult to dismiss.
Obesity is not the sole offender. The Western diet, high in ultra-processed foods, red meat, added sugars, and low in fiber, is being put under extreme scrutiny. Conjugated with alcohol intake, smoking, and a lack of physical activity, this diet could be fueling an insidious epidemic.
Another new piece of the puzzle is a toxin named colibactin, which is made by a strain of E. coli living in the colon. It has been discovered to cause damage to colon cells' DNA, forming a biological pathway that may lead to cancer formation.
Although still an emerging field of study, the presence of colibactin-producing bacteria may partly account for why some individuals with no such family history or apparent lifestyle risk develop colorectal cancer in their 30s or 40s. Researchers are increasingly looking to the gut microbiome, an intricate system heavily controlled by diet, antibiotics, and environment.
Why the American Diet Is Being Blamed for Rising Colon Cancer Rates?
The standard American diet—widely known as a Western-style diet—is rich in ultra-processed foods, red and processed meats, added sugars, and refined carbohydrates but poor in fiber, whole grain, and fresh vegetables and fruits. This dietary pattern has for a long time been linked to chronic diseases such as obesity, type 2 diabetes, and heart disease, but increasing evidence now establishes a clear connection with early-onset colorectal cancer as well.
One of the most important factors is obesity, which is growing very fast in America and is now thought to be a major driving force in the incidence of colon cancer in younger adults. Obesity causes chronic inflammation and high levels of insulin in the body—both of which are known to stimulate cancer cell growth, including within the gastrointestinal system.
In a 2019 report, obese women were found to have almost twice the risk of developing early-onset colorectal cancer. Considering that nearly half of all American adults are projected to be obese by 2030, this is an extremely troubling trend.
Furthermore, low-fiber diets prevalent in fast food-dominated and processed food-based diets prolong digestion and inhibit healthy cell turnover in the colon. Fiber is essential for a healthy gut microbiome, the disruption of which can lead to the proliferation of harmful bacteria such as E. coli. Certain strains of E. coli synthesize colibactin, a toxin that has been proven to induce DNA damage in colon cells, causing it to initiate cancer development.
The issue is not limited to a single food category, but instead a combination of unhealthy dietary habits, sedentary lifestyle, and higher intake of pro-inflammatory and metabolic-disturbance-promoting food items.
Simply put, the American diet fosters a setting where cancer is more likely to develop—making it one of the most modifiable risk factors in the fight against early-onset colorectal cancer.
Why Are Young Adults Facing the Brunt of Colon Cancer?
What's of particular concern is how quickly things are shifting. Adults born in 1990 are twice as likely to get colon cancer and four times as likely to get rectal cancer as adults born in 1950. And this increase isn't limited to America—these same trends are being seen across the world.
In American men below 50, colorectal cancer is currently the number one cause of cancer death. In women aged between 50 and younger, it comes second.
These are figures calling for action—but action is slow, in part because colorectal cancer has traditionally been viewed as an old-age disease. That presumption is causing warning signs to go missed, particularly in younger patients who are not considered at risk.
Is Being Diagnosed Too Late Making Treatment Harder?
For younger patients, the path to a diagnosis is typically riddled with obstacles. Symptoms such as abdominal pain, fatigue, rectal bleeding, or unexplained weight loss are commonly dismissed or mislabeled as less severe conditions like hemorrhoids or irritable bowel syndrome.
By the time they are diagnosed with cancer, it's usually at a late stage—decreasing treatment choices and chances of survival. Even when the treatment is given early in life, younger patients tend to receive harsh therapies, which don't always benefit them but usually compromise quality of life, including fertility, body image, and mental well-being.
As lead author Dr. Char notes, “We’re seeing patients not only battling the disease but also facing financial stress, work disruptions, and emotional trauma at a point in life where they’re just beginning careers or raising young families.”
Colorectal cancer is not affecting all communities equally. Data shows that Black, Hispanic, Indigenous, and Asian Americans are disproportionately impacted—both in incidence and mortality.
This mirrors historical inequities in access to healthcare, preventive tests, and diet, only intensified by economic inequality. In several of these populations, obstacles such as lack of trust in the medical system, lower rates of insurance, and fewer sources of healthy food create prevention and early detection much more difficult.
Public health leaders contend that to stem colorectal cancer is to address not only healthcare systems, but also the wider social determinants of health.
Screening and Awareness Need to Shift Towards Improved Prevention
The increasing burden of early-onset colorectal cancer is forcing health systems to reconsider screening recommendations and public awareness programs. The U.S. Preventive Services Task Force already reduced the recommended screening age from 50 to 45, but most experts indicate that may not be soon enough—particularly for individuals at high risk.
Vigilance raising population awareness regarding symptoms, family history risk, and changes in lifestyle is of utmost importance. Early application of screening tests such as colonoscopy and stool DNA tests is effective. However, for younger patients, particularly those who are not insured, access continues to be an issue.
Increased research on early detection, non-invasive diagnostic modalities, and individualized screening intervals is needed urgently.
The rise in early-onset colorectal cancer is not a mystery without leads. Obesity, poor diet, microbial changes, and delayed diagnoses are all contributing factors that can be addressed with policy, education, and targeted intervention but it requires collective will. That means:
- Health systems taking young patients’ symptoms seriously
- Researchers accelerating efforts into microbiome and genetic testing
- Communities pushing for better food access, active lifestyles, and equitable care
- People getting educated on their risk—and fighting for screening
This isn't simply about cancer numbers. It's about the actual lives—lives of young people—lost to a preventable, curable disease. If we persist in viewing colon cancer as something that happens to older people, we'll be losing a generation that's already up against its toughest battle yet.
© 2024 Bennett, Coleman & Company Limited

