- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Children Getting Fewer Vaccine May Be A 'Better Thing', Says RFK Jr, As US Struggles With Rise In Flu Activity

Credits: Wikimedia Commons
"May be it is a better thing that fewer children are receiving the flu vaccine," said Robert F Kennedy Jr, the Health and Human Services Secretary of the United States as the country fights with a surge in flu cases. RFK Jr said this in a response to a CBS News journalists' concern over rising flu cases and the drop in the number of flu vaccines.
This week, the Centers for Disease Control and Prevention (CDC) announced vaccines to fight respiratory syncytial virus or RSV, meningococcal disease, flu, and COVID are the only recommended jabs for children at high risk of serious illness or must be administered after being consulted with doctors and parents. This is part of the CDC and Trump administration's effort to scale back the childhood vaccine scheme.
In an interview with the CBS News, RFK Jr. said, "We are not taking vaccines away from anybody. If you want to get the vaccine, you can get it. It is going to be fully covered by insurance, just like it was before." However, now, there is an added step. Vaccines that were compulsory before now requires a consultation with the physician first. "You have to, yeah, you need to do shared decision making with your physician, which is how it ought to be," he said.
Fewer Flu Vaccine Could Be A Better Thing: TFK Jr, Is There Evidence?
The CBS News reporter asked if there is any evidence that fewer kids getting the vaccine is actually a "better thing" The reporter also pointed out that many kids died of flu last year and that no evidence shows that the kids who died were vaccinated. In fact, a roughly 90% of kids who died from the flu in 2024 were not vaccinated, showed the CDC data.
To this, Kennedy quoted a Cochrane Collaboration study. It is a UK-based health care research nonprofit, which he called, "one of the ultimate arbiters of vaccine safety and clinical data". As per him, the study is an extensive meta review of the flu vaccine. Kennedy said that the study found "there is no evidence that the flu vaccine prevents serious disease or that it prevents hospitalizations or death in children.
However, the medical community does not respect this. In fact, the CDC itself has cited from various studies, including a 2020 and 2017 study from Pediatrics journal, and a 2022 and 2014 study from the journal of Clinical Infectious Diseases, which is an official publication of Infectious Diseases Society of America, and found that vaccines in fact prevented severe and life-threatening complications in children from the flu.
The CDC notes: "ACIP recommends any licensed, age-appropriate flu vaccine for children without preference for any one flu vaccine over another. In several studies, flu vaccine effectiveness was higher among children who received two doses of flu vaccine the first season that they were vaccinated (as recommended) compared to "partially vaccinated" children who only received a single dose of flu vaccine."
However, Kennedy is fixed at his opinion that "here is no scientific evidence that the flu vaccine prevents serious illness, hospitalizations, or death in children."
Unvaccinated Traveler Triggers Measles Outbreak in US, 17 Infected: CDC
Credit: Canva
The US Centers for Disease Control and Prevention (CDC) has stated that an unvaccinated person who traveled to the US from Europe spread measles to 17 others in the country last year.
In a paper published in The Journal of Infectious Diseases, the CDC highlighted the case of an unvaccinated traveler who arrived at the Denver International Airport in Colorado in May 2025.
The person traveled with a fever, persistent cough, cold-like symptoms, and conjunctivitis (“pink eye”). He stayed overnight in a hotel and then boarded another flight to North Dakota. A day later, the person developed a rash.
“The index case was in an unvaccinated adult. Aircraft contact investigations identified 135 exposed domestic travelers. Fifteen secondary cases were identified among people exposed during the international (5) and domestic (3) flights, and at the airport (7),” the CDC said in the paper.
“Two tertiary case-patients were also identified. Five of the secondary case-patients had at least one documented prior measles vaccination,” it added.
The 2025 US Measles Outbreak
While measles was declared eliminated in the US in 2000, and sporadic outbreaks were controlled quickly, falling vaccination rates, especially during the COVID-19 pandemic, raise the risk of larger, harder-to-contain outbreaks.
This was further compounded by the anti-vaccine stance of President Donald Trump and his Health Secretary Robert F Kennedy Jr.
As per the CDC, a total of 2,281 confirmed measles cases were reported in the US in 2025. In 2026, the agency reported 10 new outbreaks, with more than 1,000 measles cases confirmed to date. More than 90 percent cases each year occurred in the unvaccinated.
Air Travel Increases Measles Spread
Recently, two passengers from India infected with measles landed in Auckland, New Zealand, via Singapore Airlines.
The Straits Times quoted Associate Professor Lim Poh Lian, group director of the Communicable Disease Agency’s (CDA) Communicable Disease Program, who noted that the individuals developed symptoms only while onboard the flight from Singapore to Auckland.
“Measles transmission may occur during travel. Travelers with fever and other overt signs of transmissible illness, such as coughing or malaise, should be strongly encouraged to delay travel while symptomatic,” the US CDC said.
Vaccination Key To Tackling Measles
Measles is a highly infectious disease characterized by the three Cs:
- Cough
- Coryza or runny nose
- Conjunctivitis or red and watery eyes
It easily spreads from one infected person to another through breaths, coughs, or sneezes and could cause severe disease, complications, and even death.
Even though a safe and cost-effective vaccine is available, in 2024, there were an estimated 95,000 measles deaths globally, mostly among unvaccinated or under-vaccinated children under the age of 5 years, according to the World Health Organization (WHO).
The CDC recommends that all travelers aged 6 months or older get vaccinated before international travel.
Rajshri Deshpande’s Breast Cancer Diagnosis Highlights Importance of Routine Screening
Credit: Instagram
Sacred Games actress Rajshri Deshpande today informed of being diagnosed with grade 1 breast cancer and shared her journey of recovery.
In an Instagram post, the 43-year-old actress announced being diagnosed with the Infiltrating ductal carcinoma (NOS) -- the most common breast cancer type, accounting for roughly 80 percent of cases.
Importantly, the actress known for her work in Trial by Fire and Manto said that the deadly disease was detected during routine screening, initiating the road to early recovery.
“As you’re reading this, it means I’ve finally found the courage to tell my parents that I have been diagnosed with Infiltrating ductal carcinoma (NOS), a grade 1 Breast cancer. Now it’s time you all know,” the Instagram post read.
“We fortunately caught this early in a routine checkup, which gave us a fighting chance,” she added.
Rajshri called her treatment with ‘tons of tests and surgery” “a rollercoaster ride”.
“Trust me, it was everyone’s love and warmth that carried me through,” she said, while thanking her fans and her parents whose “faces after surgery melted my fears into unbreakable strength”.
“With everyone’s support, I feel am ready to take on the world,” Rajshri said, adding that she “is recovering beautifully and soon heading home from the hospital”.
What Is Infiltrating Ductal Carcinoma (IDC)
Also known as Invasive ductal carcinoma, the cancer occurs when abnormal cells growing in the lining of the milk ducts change and invade breast tissue beyond the walls of the duct.
Breast ducts are the passageways where milk from the milk glands (lobules) flows to the nipple.
Common symptoms of IDC include
- A lump or thickening in the breast or armpit area
- Changes in the size, shape, or appearance of the breast
- Nipple changes, such as inversion, discharge, or scaling
- Breast pain or discomfort
- Skin changes on the breast, like redness or dimpling
Key risk factors of IDC include
- Genetic mutations such as BRCA1 and BRCA2 gene mutations, and a family history of breast cancer
- Higher levels of estrogen
- Prolonged exposure to ionising radiation
- smoking
- excessive alcohol consumption
- obesity
- a sedentary lifestyle
Rising Early-Onset Cancers in women
The recent The Lancet Oncology study mentioned a rise in new cases in women aged 20-54 years (up 29 percent) since 1990.
Recently, American actress Christy Carlson Romano announced a positive cancer screening test.
Cancer is everywhere, said Romano, 41, in a tearful video on social media platform Instagram.
A 2025 study by Duke Cancer Institute in the US revealed that for women younger than 50, the risk of developing cancer is 82 percent higher than that of men, up from 51 percent in 2022.
The 2025 annual report from the American Cancer Society (ACS) also showed that cancer rates in young and middle-aged women are rising past those of men in the same age group, but especially among women under age 50.
Importance of Early Screening
As with Rajshri, catching cancer in its early stages can help individuals experience less severe symptoms, minimize discomfort, and improve overall quality of life.
Detection of cancer at an early stage can boost survival rates. It can increase the chances of successful treatment.
Common screening methods include
- Physical examination of your breasts by your doctor
- Mammography
- Breast ultrasound
- Breast magnetic resonance imaging (MRI)
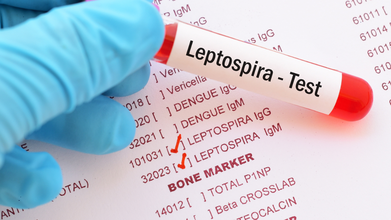
Punjab Leptospirosis Outbreak: 36 Test Positive, 19 Children Hospitalized
Credit Canva
Punjab is grappling with an outbreak of leptospirosis, a rare bacterial disease, with 36 confirmed cases, including 19 children who have been hospitalized.
The outbreak is part of a larger water‑borne infection that has affected 110 people so far in Hazara Singh Wala, a border village in Ferozepur district. The hospitalized children are receiving care at Ferozepur Civil Hospital and are reported to be stable, according to media reports.
Leptospirosis spreads to humans through contact with the urine of infected animals such as dogs and rats. The disease previously made headlines in 2024 when Punjab Chief Minister Bhagwant Mann was hospitalized after contracting it.
The state health department reports that the outbreak is affecting mainly children and young people aged 3-25, with nearly 90 of the 110 symptomatic patients being minors, most of whom are school-going children, the Indian Express reported. Commonly reported symptoms include fever, abdominal pain, and jaundice.
Alarm was heightened in the district following the death of 12-year-old Sehaj Kaur from suspected Hepatitis E on February 24.
Investigations revealed that contaminated water, including rodent droppings and dead pigeons, contributed to the outbreak, causing widespread concern among villagers.
Media reports stated that health officials in the states are taking steps to prevent further spread of the outbreak. They are conducting house-to-house screenings, distributing chlorine tablets and oral rehydration solutions, as well as repairing water supply lines to contain the outbreak.
What Is Leptospirosis
Leptospirosis is usually a disease of animals like dogs, mostly rats, and some farm animals. It has also been reported in pigs, zebras, and horses.
It mainly spreads via contamination or through direct contact with loosely available food items or water infected with rat urine.
Common symptoms include:
- very high temperature
- headache
- muscular pains
- vomiting
- diarrhea
- yellow or brown patches in the eye,
- abdomen pain
While the disease is usually self-limiting and treatable with antibiotics, in severe cases it can spread to the kidney, brain, spinal cord, and liver and lead to death. It can also cause pulmonary haemorrhage, leading to respiratory failure and death.
Leptospirosis In India:
The neglected zoonotic disease is endemic to India due to a tropical climate that complements the transmission of infection.
The first disease outbreak was reported in the 1920s in the Andaman Islands.
As per the National Centre for Disease Control (NCDC), India is expected to report 0.1-1.0 million cases per year, but less than 10,000 cases are reported.
Only four states, i.e., Kerala, Gujarat, Tamil Nadu, and Maharashtra, report more than 500 cases per year as per IDSP Disease Alert.
Andaman, Andhra Pradesh, Assam, Goa, Delhi, Karnataka, Odisha, Puducherry, and Uttar Pradesh also report cases.
Due to a lack of awareness of the disease and a lack of suitable laboratory diagnostic capabilities in most regions of the country, leptospirosis has been under-reported and under-diagnosed in India.
© 2024 Bennett, Coleman & Company Limited

