- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Deadly Virus Outbreak Triggers US Travel Warning For This Cruise Spot- Precautions Travelers Must Take
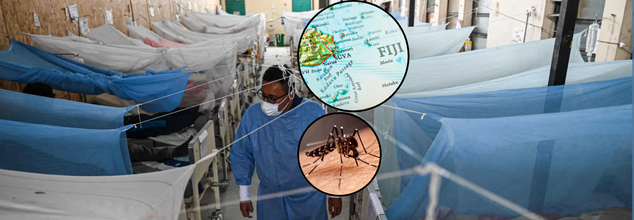
Tourists planning an idyllic South Pacific vacation in Fiji might consider putting a little something other than sunscreen into their bags, specifically protection against dengue fever, a mosquito-carried virus which is rapidly spreading across the region.
In a recent development that has alarmed global health authorities, the U.S. Centers for Disease Control and Prevention (CDC) recently issued a Level 1 travel alert for Fiji after a sharp increase in cases of dengue fever. Although the alert does not advocate for travel bans, it advises caution and attention particularly since Fiji is approaching peak cruise season and continues to receive thousands of foreign visitors.
Fiji, famous for its coral reefs, green rainforests, and untouched beaches, has also become a cruise tourist hotspot. In 2023, the island country received more than 83,000 cruise ship visitors, a figure expected to reach more than 86,000 in 2024, as per reports. But while its tourism sector blossoms, the Pacific paradise is grappling with a worrying health crisis at the same time.
The Fiji Central Division reported more than 1,000 dengue cases in January through late March alone. By the final week of February 2025, combined case reports throughout the country had climbed to 2,436. The nation's Ministry of Health credits this sudden increase with exceptionally heavy rainfall and flooding, which offer perfect breeding ground for the mosquito—the main vector for dengue virus transmission.
What is Viral Fever Outbreak In Fiji?
Dengue fever is a viral disease spread by the bite of Aedes aegypti mosquitoes. It usually develops with high fever, severe muscle and joint pain (commonly called "breakbone fever"), nausea, vomiting, severe headache, and rash. In certain instances, the person may feel pain behind the eyes—a classic symptom.
Although most cases of dengue are self-limiting, severe dengue (or dengue hemorrhagic fever) may result in life-threatening complications like internal bleeding, respiratory impairment, and organ failure.
No antiviral drug for dengue is available at present. Supportive measures like proper hydration and over-the-counter medications like acetaminophen for pain relief can be given in mild cases. Hospitalization is required in severe cases for fluid management and intensive monitoring.
What is a Level 1 Alert?
The CDC's Level 1 alert—ranked as "Practice Usual Precautions"—is the lowest on its travel health notice hierarchy. This does not, however, equate to negligible risk. Rather, it informs travelers that there is an outbreak and that preemptive action must be taken in order to not get infected.
"Spring and summer travel overlap with the high season for dengue in much of the globe, raising the possibility of both travel-related and locally transmitted cases in the United States," the CDC wrote in its latest health advisory.
Since most cruise routes go through tropical regions during these times, the chance of contact with infected mosquitoes greatly increases.
The case in Fiji is not unique. The World Health Organization (WHO) predicts that dengue has become one of the most rapidly spreading mosquito-borne illnesses in the world. As many as 100 to 400 million cases are estimated each year, and half of the world's population is now under threat.
In 2024, the World Mosquito Program had called it the "worst year for dengue on record," since outbreaks were at the same time reported in Brazil, Colombia, the Philippines, Mexico, and parts of the Caribbean. Rapid urbanization and climate change have been identified as major drivers behind this trend.
Increased global warming, higher rainfalls, and flooding—all effects of a hotter planet—provide ideal conditions for mosquito populations to flourish. "If we keep putting planet-warming gases into our air.dengue and other vector-borne diseases will only prosper," the Fiji government warned.
Precautionary Steps Tourists Need to Take Prior to and During Travel
No vaccine is universally recommended for tourists, although prevention is still the best course.
Here’s what the CDC and Fiji’s Ministry of Health recommend:
- Use EPA-registered insect repellents containing DEET, picaridin, IR3535, or oil of lemon eucalyptus.
- Wear protective clothing such as loose-fitting, long-sleeved shirts and pants, especially during dawn and dusk when mosquitoes are most active.
- Stay in accommodations with screened windows, air conditioning, or use mosquito bed nets if sleeping outdoors or in unscreened environments.
- Avoid standing water, which serves as breeding grounds for mosquitoes.
- If bitten, do not scratch the bite and use hydrocortisone cream or calamine lotion to reduce itching.
In addition, travelers should visit their healthcare provider prior to travel and remain up to date with information from the CDC, WHO, and local health authorities.
Fiji’s Ministry of Health has ramped up its vector control operations, including mosquito surveillance, fogging in affected areas, and community education. Public service announcements are urging residents and tourists alike to eliminate mosquito breeding sites and seek medical attention at the first sign of symptoms.
Cruise lines with Fijian stops have also increased measures to inform passengers about health risks and preventive measures. Unless the outbreak worsens further or spreads to other areas, cruise operations are likely to go on as usual.
Medical experts warn that dengue fever outbreaks will become more common and extensive as a result of climate changes and more travel internationally. As a result, travelers need to start considering mosquito-borne diseases not as far-off chances but as actual health factors particularly when traveling to tropical areas.
Until now, Fiji has been open to tourism, but the spike in dengue cases is a wake-up call: the loveliness of an environment that does not protect it from threats to global health. Whether on a beach or trekking through rainforests inland, simple but crucial precautions may be the difference between a vacation dreams and a medical crisis.
Adenovirus Cases Rise In UK, Health Authorities On Alert
Credits: Canva
Adenovirus, or what some people are calling the mystery disease is going to be the next worry in the UK, after superflu had already grappled the healthcare system. Along with H3N2 and its variant superclade K, people are now worrying about this mystery disease.
Adenovirus is highly contagious and causes mild cold or flu-like symptoms, though severe cases could lead to stomach flu and vomiting. Many describe this virus as 'heartier' than others. The reason is that the virus can survive longer on surfaces and even resist the common disinfectants used. This is what makes it highly transmissible.
Is There Any Treatment For Adenovirus?
As of now, there is no treatment for adenovirus, it could however be managed and monitored. What helps is regular handwashing and thorough cleaning of surfaces.
The good news is that cases of adenovirus are actually dropping in the UK, as confirmed lab reports. The cases last week were 1.2 per cent, whereas the week prior, it was at 1.7 per cent, as also reported by the Independent.
What Are The Symptoms Of Adenovirus?
While a lot of the symptoms mimics of those in flu or COVID, including shortness of breath, a sore throat and or a runny nose. However, there are certain unique symptoms of adenovirus that include:
- Diarrhea
- Pink Eye or conjunctivitis
- Ear infection or otitis media
- Swollen lymph nodes
- Pneumonia
- Stomach pain
- Nausea
- Vomiting
Other rare symptoms could also include impact on your bladder or nervous system. As viruses in your bladder can also cause urinary tract infections, and the same virus in your nervous system can cause condition that can affect your brain. These conditions also include encephalitis and meningitis.
The symptoms usually start to subside within two days, however, if the symptoms stay even after three days without any relief, it might be a red flag. The best thing to do during such a situation is to go consult your GP.
Also Read: Unique Symptoms Of Mysterious Adenovirus And How Long Infection Now Last
Is Adenovirus Spreading Faster Than Flu and Covid?
Experts have noted that due to its ability to stay longer in the environment and being highly transmissible, it is in fact, spreading faster than flu and COVID. Eric Sachinwalla, Jefferson Health's medical director said that this virus is still unfamiliar and thus not much can actually be done to treat adenovirus. Speaking to PhillyMad, he said, "It is pretty contagious because it is heartier than other viruses - soap and water, or everyday disinfectant, won't kill it, so it tends to live in the environment longer."
What Are The Ways To Stay Safe From Adenovirus?
Since adenovirus spreads through close contact and is resistant to many everyday disinfectants, hygiene remains the key. The best way to stay safe is by avoiding close contact, especially with those who are unwell. You may also keep an eye on your symptoms, including your body temperature and take steps to prevent the virus from spreading by taking precautions, as well as getting the flu jab.
WHO Approves Two COVID-19 Rapid Antigen Tests, Check The Details Here

Credits: iStock
The World Health Organization (WHO) on December 24 prequalified two rapid antigen diagnostic tests (Ag-RDTs) for SARS-CoV-2. This virus is known to have caused COVID-19. As per the WHO, these two tests are called SD Biosensor STANDARD Q COVID-19 Ag Test and the ACON Biotech Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing).
At first these tests got temporary emergency approval from the WHO during the pandemic. This was done so the countries could start using them quickly even though long-term data was limited. This emergency approval helped the tests reach over 100 countries when they were urgently needed.
What Has Changed Now?
The WHO has now given these tests full prequalification, which means it has a stronger and long-term approval. This means that the WHO has thoroughly checked and confirmed that the tests consistently meet global standards for quality, safety, and accuracy.
Why Does This Matter?
- The tests can now be officially bought and used by global organizations and governments
- They can be included in bulk purchasing to lower prices and increase supply
- This makes reliable COVID-19 testing more affordable and accessible, especially in low-and middle-income countries
COVID-19 Is Far From Being Over
Even though WHO officially ended COVID-19 emergency phase over two years ago, the virus is still circulating globally. In fact, this year, we have seen variants of COVID-19 circulating around, causing the most unique symptoms, including razor-blade like throat. Variants like JN.1, Stratus, Nimbus, LP8.1, and BA.3.2 were all that we saw in 2025.
While the good news is that infection levels are relatively stable, but the virus has not completely disappeared and testing is still necessary, especially in poorer countries.
Many low-income countries do not have easy access to labs or expensive PCR testing. So there is still a need for a strong, but cheaper and reliable way to detect COVID-19, and these tests may as well do that.
Why Rapid Antigen Test Matters
- Give results in 15–30 minutes
- Cost less than PCR tests
- Don’t require labs or specialized equipment
- This means they can be used in small clinics, community centers, mobile vans, or remote areas, where lab testing isn’t practical.
However, it is important to note that rapid antigen tests are not replacement for PCR tests. They simply complement the PCR tests by allowing faster, on-the-spot decisions, especially when the lab capacity is limited.
What Makes It Essential Today?
Rapid antigen tests could help with spotting and stopping local outbreaks quickly, protecting high-risk people and healthcare workers, and staying prepared for future respiratory pandemics.
The WHO is also pushing for decentralized, quality-checked testing as part of universal healthcare and global health security, so countries aren’t caught unprepared when the next outbreak happens.
When Is It A Right Time To Get Yourself Checked For COVID-19?
If you notice these following symptoms as noted by the Centers for Disease Control and Prevention (CDC), it is best that you get yourself a COVID-19 test:
- Fever or chills
- Cough
- Shortness of breath or difficulty breathing
- Sore throat
- Congestion or runny nose
- New loss of taste or smell
- Fatigue
- Muscle or body aches
Singapore, UK and Canada Issue Health Advisory Over Delhi’s Air Pollution

Credits: iStock
As Delhi's air quality levels and pollution continues to worsen, and people struggle to breathe, countries like Singapore, the United Kingdom, and Canada issued advisories for their citizens travelling to the capital city. However, the capital city's crisis continues to remain ignore, though CM Rekha Gupta did hold a review meeting on the same on Monday, with a follow-up scheduled on Thursday.
Singapore Travel Advisory On Delhi's Air Pollution
Singapore High Commission issued an advisory, which stated:
On 13 December 2025: The Indian Central Pollution Control Board invoked Stage 4, the highest level, of the Graded Response Action Plan (GRAP) in the Delhi National Capital Region. Under GRAP 4, construction and industrial activities are heavily restricted, and schools and offices are encouraged to shift to hybrid format. The Delhi authorities have urged residents to stay indoors, especially children and those with respiratory or cardiac ailments, and to use masks if stepping out. In this regard, the High Commission urges Singapore nationals in the Delhi National Capital Region to pay heed to this advice.
We also note that given the low visibility, flights to and from the Delhi National Capital Region are likely to be affected. The Indira Gandhi International Airport and several airlines have issued advisories. Travellers should take note of this, and check with the respective airlines for updates.
UK And Canada Too Issue Advisories On Delhi Pollution And Its Impact On People
UK's Foreign, Commonwealth & Development Office (FCDO) warned that air pollution could lead to serious health threats for those living in northern India, especially between the months of October and February. The UK advisory also stressed on the health of pregnant women and people with any heart or respiratory. The advisory noted that they must seek medical advice before travelling to India.
The statement read: Children, the elderly and those with pre-existing medical conditions may be especially affected. If you’re pregnant or have a respiratory or heart condition you may wish to consult a medical practitioner before you travel.
Canada too issued a similar notice, advising, especially those who are already struggling with respiratory issues and to continuously monitor air quality levels. The notice also highlighted the pollution that is caused by fog and smoke trends that are increasing in urban areas like Delhi, especially during the winters.
The advisory read: Smoke haze and other types of air pollution can be extremely hazardous in urban areas and cities such as Delhi. It’s typically at its worst in winter. In rural areas, air quality can be affected by agricultural burning. Dust storms also occur across northern India. Monitor air pollution levels, which change quickly, especially if you suffer from respiratory ailments or have pre-existing medical conditions.
As of today, Delhi's air quality remained in "very poor" category, and the AQI stood at 342 at 8am, as per the Central Pollution Control Board. The 24-hour average AQI was logged at 412 under the "severe" category on Tuesday evening when Delhi's quality peaked to record the fourth severe air day in the month of December.
© 2024 Bennett, Coleman & Company Limited

