- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Ebola Outbreak: Uganda Set To Start Vaccine Trials

On Thursday, Uganda confirmed an outbreak of the Ebola virus in its capital city Kampala, with the first confirmed patient dying from it a day before. As per the new developments, the officials are now preparing to deploy a trial vaccine to put an end to this outbreak.
Groups of scientists are working on the vaccine and deployment of more than 2,000 doses of a candidate vaccine against the Sudan strain of Ebola has been planned and confirmed by the Uganda Virus Research Institute. As per the World Health Organization (WHO), Uganda has access to 2,169 doses of trial vaccine. For now, however, there are no approved vaccines for the strain and officials are still investigating the source of the outbreak.
The WHO had also allocated $1 million from its contingency fund for emergencies to support quick action and contain the outbreak in the country.
Confirmed Case
On Wednesday, the Sudan strain of Ebola killed a nurse employed at Kampala's main referral hospital. It is after his death that Ebola was declared an outbreak in the country. Post-mortem samples too have confirmed the Sudan Ebola Virus Disease and at least 44 contacts of the deceased man have been listed for tracing. 30 of these are health workers.
Ebola is a highly infectious hemorrhagic fever, which is transmitted through contact with bodily fluids and tissue. Symptoms include headache, vomiting of blood, muscle pains and bleeding.
it was in the late 2022, when Uganda had last suffered an Ebola outbreak. It killed 55 of the 143 people who were infected and was declared over on January 11, 2023.
What Is Ebola Virus Disease?
As per the WHO, Ebola virus disease (EVD) is a rare but severe illness in humans and is often fatal. People can get infected with the virus if they touch an infected animal when preparing food, or touch body fluids of an infected person such as saliva, urine, faeces or semen, or things that have body fluids of an infected person like clothes or sheets.
How Does Transmission Work?
Ebola enters the body through cuts in the skin or when one is touching their eyes, nose or mouth. Early symptoms include fever, fatigue and headache.
It was first discovered in 1976 in two simultaneous outbreak, when in Nzara, South Sudan and other in Yambuku, Democratic Republic of Congo. The latter occurred near a village near the Ebola River, which is where it gets its name from.
It is highly infectious and transmissible disease, in fact, there have been cases of health-care workers who have frequently been infected while treating patients with suspected or confirmed Ebola. This occurs through close contact with patients when infection control precautions are not practiced strictly.
Cases of people conducted burial ceremonies, involving direct contact with the body of the deceased too can lead to the transmission of Ebola. Even after the long suffering and recovery, there is a possibility of sexual transmission. Pregnant women who get acute Ebola and recover may still carry the virus in their breastmilk, or in pregnancy related fluids and tissues.
Symptoms:
- feeling tired
- headache
- muscle and joint pain
- eye pain and vision problems
- weight gain
- belly pain and loss of appetite
- hair loss and skin problems
- trouble sleeping
- memory loss
- hearing loss
- depression and anxiety
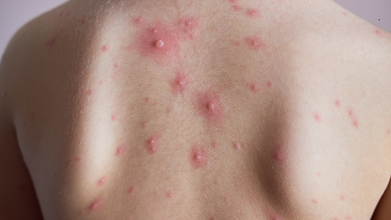
New Monkeypox Variant Emerges as Two Strains Combine, WHO Warns
Credit: Canva
Two strains of the monkeypox virus (MPXV) have combined with each other to create a new version of the disease, prompting the World Health Organisation to raise an alarm.
According to scientists, the Ib and IIb of MPXV have mutated together, and a case has been found in the UK and India, respectively. The first case was detected in the UK with travel history to a country in South-East Asia, and the second in India, with travel history to a country in the Arabian Peninsula.
Further analysis of each case shows that the two individuals fell ill several weeks apart with the same combined strain. Both cases had similar reported signs and symptoms of the disease and neither experienced severe outcomes.
As of now, these are the only known cases of this version of the virus.
What Is Mpox?
Mpox (formerly monkeypox) is an infectious disease caused by the monkeypox virus, a member of the Orthopoxvirus genus. Symptoms usually appear 1-21 days after exposure, and the illness lasts 2–4 weeks. People are considered to be contagious until all scabs have fallen off.
Mostly based in Africa, it was discovered by captive monkeys in 1958, after whom the disease was named in 1970. However, the name attracted many racist comments, especially on social media, where people wrote “the disease of monkeys” and associated it with Africans.
Under WHO guidelines, the naming of diseases must not drive any unnecessary negative impact on trade, travel, tourism or animal welfare, and avoid offending any cultural, social, national, regional, professional or ethnic groups. Thus, the name monkeypox became the ‘m-pox’.
How Can You Detect Mpox?
There are signs and symptoms of M-pox. They start to show within seven to 14 days of being infected. Therefore, for about a week, a person may not know they have m-pox, and they can spread it by travelling.
The earliest signs are getting a fever, sweating and having chills through your body. Other signs involve rashes, which start from a distant rash on the face and spread throughout the body. These rashes can be in different forms, sometimes a flat lesion, bumps, boils or scabs.
Symptoms can also include swollen lymph nodes, migraine, muscle aches, fatigue, weakness and back pain.
How Do You Prevent Mpox?
This is a contagious infection and can spread by skin-to-skin contact. Here are some things you can do to safeguard yourself:- Restrict your movement by avoiding going out in public, meeting people and interacting with animals.
- Wear clothes that will prevent skin-to-skin infections.
- Hydrate yourself to get rid of the toxins from your body
- Check if you have received your smallpox vaccination
Doctors also prescribe medications like acetaminophen and ibuprofen to treat the pain and fever one may experience after being infected
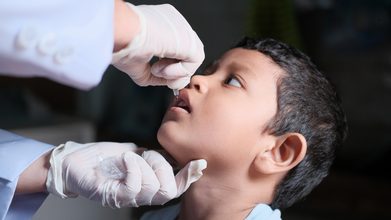
WHO Approves New India-Origin Oral Polio Vaccine for Outbreak Fight
Credit: Canva
The World Health Organization (WHO) has prequalified an additional novel oral polio vaccine type 2 (nOPV2) to strengthen the global supply of the vaccine, stop poliovirus type 2 outbreaks more sustainably and accelerate progress towards polio eradication.
The newly approved vaccine was manufactured by Biological E. Limited (BioE), Hyderabad, India, using in-house bulk vaccine following a technology transfer from PT Bio Farma (Persero), Indonesia.
The prequalification status means that the vaccine meets international standards of quality, safety, and efficacy for global immunization programmes.
It also allows the vaccine to be purchased and supplied through United Nations procurement agencies, including UNICEF, across the world and helps governments prevent and control poliovirus transmission.
With the new WHO recommendation, BioE is expected to produce 600 million doses per year of the nOPV2, increasing India's global leadership in vaccine manufacturing and its contributions to expanding access to affordable life‑saving vaccines across the Global South.
WHO Director-General Dr Tedros Adhanom Ghebreyesus highlighted the impact of vaccination efforts on global polio eradication and said: “Vaccines are also bringing us closer to the eradication of polio, with 41 cases of wild polio reported last year from just 24 districts in Pakistan and Afghanistan, down from 99 cases in 49 districts in 2024."
What Is nOPV2?
Novel oral polio vaccine type 2 (nOPV2) is a genetically modified, more stable vaccine designed to stop circulating vaccine-derived poliovirus type 2 (cVDPV2) outbreaks. It provides comparable protection to the old type 2 vaccine (mOPV2) but is less likely to cause new vaccine-derived outbreaks.
Studies show nOPV2 is safe, well-tolerated and produces similar immunity to mOPV2, with over 500 million doses administered across 23 countries as of early 2024. The vaccine is typically used in targeted, large-scale vaccination campaigns, often for children under five years of age.
The oral vaccine is supplied in 20-dose and 50-dose vial presentations. It has a shelf-life of 24 months when stored at temperatures not exceeding –20 °C and can also be stored for up to six months at +2 °C to +8 °C, providing important flexibility for immunization programmes in diverse situations.
Mike McGovern, Chair of the International PolioPlus Committee, Rotary International and Chair of the Polio Oversight Board, GPEI, said of the new prequalification: "Expanding nOPV2 manufacturing is essential to ensuring countries can respond quickly to variant poliovirus outbreaks. Biological E’s prequalification status strengthens the global supply and brings us closer to ending these outbreaks for good."
Why Is The New Polio Vaccine Useful?
Once a global scourge paralysing hundreds of thousands of children globally, polio is now close to eradication as wild poliovirus cases have fallen by more than 99 percent since the 1980s.
However, given rising vaccine hesitancy and gaps in immunisation, cVDPV2 can re-emerge in under-immunised communities, triggering outbreaks as seen recently in parts of Africa and Southeast Asia. But nOPV2's genetic stability and increased protection help limit that risk and reduce the chances of a mass outbreak.
While India achieved its polio-free certification in 2014, the risk of importations or vaccine-derived outbreaks increases if coverage drops. With the nOPV2's WHO prequalification, officials can safeguard public health by ensuring access to the most advanced tools available.
As of August 2022, 18 out of 21 countries had successfully stopped cVDPV2 transmission following two rounds of mass vaccination activity and a further two countries did so after a third round.
Data has shown that nOPV2 effectiveness is on par with that of mOPV2, and while evidence of the vaccine’s immunogenicity has already been well established, additional studies continue to be conducted throughout the EUL period to confirm protection against type 2 poliovirus in vaccinated individuals.
Mizoram Officials Call Rising HIV Cases 'Collective Disgrace', Launch Love Brigade 2.0

Credit: NE Now
Mizoram Health Minister Lalrinpuii has expressed serious concern about rising HIV cases, as data shows that cases in the state are 13 times higher than the national average.
Calling it a "collective disgrace", Lalrinpuii said: “About 70 per cent of HIV transmissions in Mizoram occur through sexual contact. While the national prevalence rate stands at a mere 0.2 per cent, Mizoram’s rate has climbed to 2.74 per cent. This is a matter of shame for Mizo society.”
Speaking at an event organised for International Condom Day in Aizawl on February 13, she noted that a majority of that sexual contact remains the primary route of HIV infections in the state, accounting for 70 per cent of all cases.
The minister noted that the spread is largely driven by infidelity and a lack of preventive measures, which she argued contradicted the moral and religious values of the Mizo society.
She urged the people of the state to remain faithful to their partners. “To protect the future of Mizoram, our youth must remain vigilant,” Lalrinpuii added.
The global challenge of HIV/AIDS remains one of the most pressing public health issues today. According to the latest data from UNAIDS, around 38.4 million people worldwide are living with HIV/AIDS.
As of 2024–2025, India has approximately 2.5 million to 2.56 million people living with HIV (PLHIV), marking the third-largest HIV epidemic globally, underlining the need for not only medical intervention but also comprehensive awareness, education, and social change.
How Does Mizoram Plan To Reduce HIV Cases?
During the event, Laltinpuii said condoms are recognised globally as one of the most effective and accessible tools for preventing HIV as well as other sexually transmitted infections, urging the public to discard misconceptions surrounding use.
While suggesting abstinence before marriage, the minister also advised sexually active individuals not to hesitate to use condoms, as they play a vital role in preventing the spread of the virus, if used correctly.
“While we advocate abstinence before marriage, those who cannot abstain must not hesitate to use condoms. It is a simple, life-saving tool that we must stop stigmatising,” she said.
During the event, Laltinpuii launched the “Love Brigade 2.0” campaign aimed at normalising condom access across the state. Under the initiative, two-wheeler taxi riders have been equipped with “Love Brigade Condom Jackets”, allowing passengers and members of the public to access free condoms discreetly and conveniently.
What Is HIV?
HIV is the virus responsible for attacking the body’s immune system, specifically targeting CD4 cells, which are crucial for the body’s defense against infections. As HIV progresses, it destroys these cells, weakening the immune system over time. If left untreated, this continuous damage can lead to AIDS.
HIV is highly contagious and can be transmitted through the exchange of bodily fluids such as blood, semen, vaginal fluids, and breast milk. It is primarily spread through unprotected sexual contact, sharing needles, from mother to child during childbirth or breastfeeding.
Without treatment, HIV progresses through three stages:
- Acute HIV Infection: This stage occurs shortly after transmission and may include symptoms like fever, fatigue, and swollen lymph nodes.
- Chronic HIV Infection: Often asymptomatic or mildly symptomatic, the virus continues to damage the immune system but at a slower rate.
- AIDS: This is the final stage, marked by severe immune damage and the presence of infections that take advantage of the compromised immune defenses.
The disease is diagnosed through blood tests or oral swabs that detect the presence of the virus or antibodies produced by the immune system in response to the virus. Early detection of HIV is crucial, as it allows for timely intervention and treatment, which can prevent the virus from progressing to AIDS.
For HIV, the primary treatment goal is to suppress the virus to undetectable levels, thus maintaining a strong immune system and preventing further transmission of the virus. People living with HIV can often live long, healthy lives if they adhere to antiretroviral therapy (ART).
© 2024 Bennett, Coleman & Company Limited

