- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Eli Lilly’s Weight Loss Pill Advances In First Late-Stage Trial; How Does This Redefine Global Health?

Eli Lilly's newest breakthrough is a oral weight loss pill, having passed its initial late-stage test, it may revolutionize the way we think about health care, especially in the fight against obesity and diabetes.
In a development just announced that promises to change the future of managing chronic diseases, drugmaker Eli Lilly has said its oral weight-loss medicine, orforglipron, has attained its main objectives in the first of multiple late-stage clinical trials. The study, in patients with Type 2 diabetes, showed that the pill can reduce both blood sugar and body weight successfully without injections.
As demand picks up for GLP-1 medicines such as Novo Nordisk's Ozempic and Wegovy, Eli Lilly's orforglipron joins a high-risk competition to provide effective, easy-to-use alternatives to injectable drugs. The advantage of orforglipron over its rivals is that it is not a peptide-based medicine. What that implies is that it is simpler for the body to absorb and is not accompanied by dietary limitations common with oral GLP-1 medicines such as Novo's Rybelsus.
The medication works by simulating incretin hormones—naturally occurring gut hormones such as GLP-1 that control blood glucose and curb appetite. By making this available in pill form, Lilly is positioning orforglipron as a strongly scalable, easier-to-produce, and more patient-friendly alternative that doesn't need refrigeration or injection pens—a particularly significant transition for global health accessibility.
In its initial late-stage trial, orforglipron allowed Type 2 diabetes patients to lose a 7.9% decrease in body weight over 40 weeks, which averaged about 16 pounds. Notably, there was no weight loss plateau at the conclusion of the study, suggesting the possibility of even greater outcomes over time.
But while the weight-loss numbers were strong, the pill came up a little short of expectations in one key diabetes measure. Hemoglobin A1c—measure of blood sugar—fell by between 1.3% and 1.6% by dose, versus a 0.1% fall in the placebo group. While notable, this was below the 1.8% to 2.1% fall some analysts had expected, particularly in comparison with current injectables such as Novo's Ozempic.
However, Eli Lilly is still hopeful. "Our new incretin medicine has met our expectations for safety and tolerability, glucose control and weight loss," CEO David Ricks said. "We anticipate further data readouts later this year."
The side effect profile of orforglipron is most in line with the side effects of injectable GLP-1 medications. The most frequently reported side effects were nausea (16%), diarrhea (26%), and vomiting (14%), but all were mild to moderate. Approximately 8% of the highest-dose group dropped out of treatment due to side effects—within the range analysts predicted.
Notably, no liver safety issues were reported—an particularly comforting finding considering Pfizer's recent abandonment of its own oral GLP-1 candidate due to liver problems encountered during trials. The lack of severe adverse events has further reinforced confidence in the safety of orforglipron and encouraged hope for its prospects in regulatory consideration.
How Is Orforglipron Different?
What distinguishes orforglipron isn't merely its effectiveness—it's the convenience and adaptability it offers. In contrast to Rybelsus, which demands fasting prior to use and food restriction, orforglipron may be used without dietary restrictions. And although tirzepatide (Lilly's injectable drug called Mounjaro) has been slightly more effective, that orforglipron acts on only one hormone and still closely approaches results is significant.
This places orforglipron firmly in contention for those patients who want a less invasive, day-to-day solution to their weight and blood sugar management that doesn't sacrifice much in terms of performance.
Eli Lilly's five-year strategy is bold. With seven late-stage trials in progress—five for diabetes and two for obesity—the firm will file for obesity treatment FDA approval by the end of 2025 and for Type 2 diabetes in 2026.
Outside of diabetes and obesity, Lilly is investigating other disease uses. For hypertension and other diseases where patients want oral therapies, the pill is being studied, says Lilly's Chief Scientific Officer, Dr. Dan Skovronsky. That opens up the potential for orforglipron to be used in preventative medicine, allowing patients to control risk factors such as high blood sugar or weight before chronic disease sets in.
The significance goes beyond the clinical—they're financial. Industry experts estimate the market for GLP-1 by the early 2030s at well over $150 billion, oral versions contributing perhaps around $50 billion. To Eli Lilly, with also newly winning FDA clearance of Alzheimer's candidate Kisunla and exploring gene therapy, orforglipron adds to its portfolio still another hoped-for blockbusted nominee.
If approved, orforglipron would be the first oral GLP-1 weight-loss drug, putting Lilly in a dominant position in a growingly competitive space.
Most appealing, perhaps, is orforglipron's potential to reshape society's view of weight management. Such drugs already have been associated with reduced risk for heart disease, sleep apnea, and even addiction and Alzheimer's, due to their metabolic and anti-inflammatory actions.
If used extensively, drugs such as orforglipron may bring about radical changes in consumer behavior, such as altered food demand. Already, some markets are showing decreases in the sale of high-calorie or sugary snacks, a development that may disrupt the diet and processed food industries.
Although orforglipron is still pending regulatory approval, preliminary data has caused warranted enthusiasm. By removing injections, having a robust safety profile, and providing quantifiable gains to Type 2 diabetes patients, this one-daily tablet may transform obesity therapy—and in the process, redefine world health outcomes.
How Does Weight Loss Pill Redefine Global Health?
Eli Lilly's development of orforglipron, an oral weight loss pill, has the potential to significantly reshape global healthcare, particularly in the realms of obesity management and diabetes care. Weight loss and diabetes treatments, especially the newer generation of GLP-1 (glucagon-like peptide-1) receptor agonists like Ozempic and Wegovy, have already created waves by offering transformative results. These injectable medications have been hailed for their ability to reduce blood sugar levels in type 2 diabetes patients while also supporting significant weight loss, addressing two critical health issues simultaneously.
However, despite their efficacy, the need for injections has been a barrier for many patients, contributing to lower adherence rates and complicating long-term usage. This is where orforglipron changes the game. As an oral GLP-1 receptor agonist, orforglipron provides a more accessible, needle-free alternative that could potentially reach a much larger pool of patients worldwide. Its ease of use, requiring no injections or refrigeration, makes it a more convenient option, especially for people in regions where access to injectable medications may be limited or costly.
The impact on diabetes care is substantial, as the pill not only aids in controlling blood sugar but also promotes weight loss, an essential factor in managing type 2 diabetes. The combination of improved metabolic control and reduced weight could reduce the burden of diabetes-related complications, including heart disease, kidney issues, and even certain cancers. Furthermore, as orforglipron could be manufactured and distributed on a larger scale, it stands to offer a more cost-effective solution to obesity and diabetes, transforming health systems globally.
Top U.S. Medical Associations Ousted from CDC Vaccine Workgroups in Sudden Shake-Up

Credits: Canva
In a controversial move that has rattled the U.S. medical community, federal health officials have severed ties with more than half a dozen major medical organizations from participating in government vaccine advisory workgroups.
The decision, communicated via email on Thursday, disinvites top experts from these groups from contributing to the workgroups that support the Advisory Committee on Immunization Practices (ACIP), a key body that guides the nation’s vaccination policies.
Organizations affected include the American Medical Association (AMA), the American Academy of Pediatrics (AAP), the Infectious Diseases Society of America (IDSA), and several others, many of whom have historically played a critical role in shaping vaccine guidelines.
“This is deeply concerning and distressing,” said Dr. William Schaffner, a renowned vaccine expert from Vanderbilt University who has been involved with ACIP workgroups for decades. “Removing these organizations will likely create conflicting messages about vaccine guidance. Patients might hear one thing from the government and another from their personal doctors.”
Longstanding Collaboration Ends Abruptly
For years, the ACIP has relied on a structured system where experts from various medical and scientific fields evaluate vaccine data and help draft recommendations. These recommendations, once approved by the Centers for Disease Control and Prevention (CDC), often inform clinical practice and determine insurance coverage.
But according to an email obtained by Bloomberg and confirmed by federal officials on Friday, the medical organizations are now being sidelined on the grounds that they are “special interest groups” and are assumed to carry a “bias” due to the populations they serve.
Dr. Schaffner defended the former system, highlighting how professional organizations offered practical insights on how recommendations could be realistically implemented in clinical settings. Importantly, all members were subject to conflict-of-interest vetting, ensuring objective guidance, he added.
Health Secretary Kennedy’s Sweeping Changes
This latest shake-up follows an earlier, unprecedented move in June when U.S. Health Secretary Robert F. Kennedy Jr. abruptly dismissed the entire ACIP panel, accusing it of being too closely aligned with vaccine manufacturers. Kennedy, a former leader in the anti-vaccine movement, has since appointed several known vaccine skeptics to the new committee.
Among the organizations removed from the workgroup process are the American Academy of Family Physicians, American College of Physicians, American Geriatrics Society, American Osteopathic Association, National Medical Association, and the National Foundation for Infectious Diseases.
In a joint statement released Friday, the AMA and several of the disinvited organizations denounced the decision, calling it “irresponsible” and “dangerous to our nation’s health.” The statement warned that excluding their medical expertise “will further undermine public and clinician trust in vaccines.”
The groups urged the administration to reverse the decision, emphasizing the importance of transparency and collaboration in public health decision-making.
Lawsuit and Fallout
Several of the ousted organizations had previously criticized Kennedy’s overhaul of the ACIP. Last month, three of them joined a lawsuit challenging the government’s decision to halt COVID-19 vaccine recommendations for most children and pregnant women, a policy shift that has been widely criticized by public health experts.
Meanwhile, newly appointed ACIP member Retsef Levi, a professor of business management with no formal medical background, defended the administration's direction on social media. Levi wrote that future workgroups would “engage experts from an even broader set of disciplines,” and claimed that membership would be based on “merit & expertise, not organizational affiliations with conflicts of interest.”
The Department of Health and Human Services (HHS) has not yet disclosed which experts will replace the disinvited members or when the new workgroups will begin operating.
US Weighs China Travel Warning As Chikungunya Cases Near 5,000: Report

Credits: Canva
The United States Centers for Disease Control and Prevention (CDC) is assessing a potential travel notice for China following a sharp rise in cases of chikungunya, a mosquito-borne viral infection that has sparked public health alarms in southern China, as reported by the Independent and the South China Morning Post.
Nearly 5,200 infections have been reported in the Guangdong province since early July, with most of them concentrated in the city of Foshan. Health officials there have since escalated their emergency response to a level III alert, which signals a “relatively major” public health threat in China’s four-tier system.
While the CDC has not yet published a formal advisory, a spokesperson told The Independent that the agency is “aware of the reported chikungunya outbreak in Guangdong Province in China and is currently assessing the size and extent of the outbreak.”
What is chikungunya?
Chikungunya is a viral infection transmitted by the bite of an infected Aedes mosquito, the same mosquito species responsible for dengue and Zika. Symptoms typically include sudden onset of fever and joint pain, but may also include headache, muscle pain, swelling, and rash.
Although most cases are mild and self-limiting, some infections can lead to prolonged joint pain or, in rare cases, long-term complications. Serious outcomes are more likely among those with pre-existing health conditions. There are no antiviral treatments available, so prevention, particularly mosquito control and bite avoidance, remains the primary approach.
Vaccines against chikungunya have recently become available and are recommended for travelers to high-risk areas, although they are not yet widely accessible.
According to local health authorities in Foshan, around 95% of reported cases have been mild, with patients recovering within a week. However, the outbreak’s rapid spread has raised concern among international health bodies.
Global spread and WHO alert
The outbreak in China follows a global pattern of chikungunya resurgence. The World Health Organization (WHO) issued an alert last week warning of the risk of the virus repeating its global spread from two decades ago. Diana Rojas Alvarez, a medical officer with WHO, said that nearly 5.6 billion people across 119 countries live in areas where the virus could potentially spread.
Chikungunya was first identified in 1952 in Tanzania and has since been detected in more than 110 countries, including major outbreaks in India, Italy, and the Americas. The virus is not spread from person to person; instead, it is carried by mosquitoes that have fed on infected individuals and then pass it to others.
The concern is not just local: international travel plays a key role in how the virus crosses borders. Infected travelers returning to or visiting countries with mosquito populations capable of transmitting the virus can trigger new outbreaks.
China’s response: Mosquito control and surveillance
The first case in this outbreak was reported in Foshan’s Shunde district on July 8 and was believed to be imported. Since then, local and national health authorities have moved quickly to contain the spread.
Measures taken include the use of drones to detect rooftop water accumulation, the release of larva-eating fish into lakes, and widespread public awareness campaigns. Residents have been urged to eliminate standing water, install window screens, and wear protective clothing.
Hospitals in affected areas have increased bed capacity for confirmed cases and designated specialized treatment centres. Border controls have been stepped up in Hong Kong to prevent imported cases from mainland China, with expanded testing capabilities introduced at key entry points.
CDC travel warnings: What it means for travelers
The CDC’s travel health notices are used to inform travelers about global disease risks and provide precautionary guidelines. The warning system has four levels, ranging from “practice usual precautions” (Level 1) to “avoid all travel” (Level 4).
As of now, China has only a Level 1 travel health notice for measles. However, the CDC has issued Level 2 notices for chikungunya in several countries including Bolivia, Kenya, and Madagascar in recent months.
If the CDC decides to escalate China’s status, it would be a significant development, both in terms of travel planning and diplomatic perception.
US–China tension and public health
The potential issuance of a travel notice also comes against the backdrop of complex US–China relations. While the CDC’s move would be grounded in public health data, the optics of a travel warning could have broader implications.
On Thursday, Chinese foreign ministry spokesperson Guo Jiakun responded to the reports, saying that China is in communication with the WHO and “making every effort to ensure a safe environment for travelers.”
The WHO has not issued any travel restrictions related to the outbreak but continues to monitor the situation closely.
A word of caution for travelers
With mosquito-borne diseases on the rise globally, driven by climate change, urbanization, and increased mobility, health experts advise travelers to stay informed and take preventive measures.
“Mosquito control is key,” said an official from the Hong Kong Centre for Health Protection. “Simple actions like using insect repellent, sleeping under mosquito nets, and avoiding stagnant water can go a long way in preventing infection.”
As global health agencies monitor the chikungunya outbreak in China, travelers to affected areas should remain vigilant and stay updated with official advisories. Prevention remains the best protection in the face of a disease with no cure.
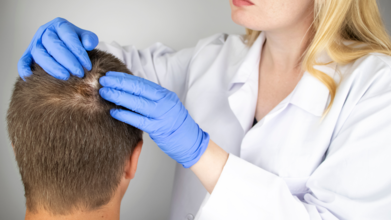
World Alopecia Day 2025: Theme, Origin, And Significance
Credits: Canva
August is known as the Hair Loss Awareness Month and the first Saturday of this month is known as the International Alopecia Day.
Hair loss is a common concern, affecting over 85% of men, 55% of women, and between 15–38% of adolescents at some point in their lives. For those with advanced or long-lasting alopecia, the emotional and social impact can be profound.
Baldness has been linked to significant declines in mental health and quality of life, with higher rates of anxiety, depression, stress, and reduced self-esteem.
What Is Alopecia?
It is a term used for hair loss that affects the scalp or even the entire body, temporarily or permanently. Alopecia can happen due to variety of reasons, including heredity, hormonal changes, and medical conditions, or as simple as normal aging.
Aim of Alopecia Day
The day aims to form a community of those who experience this autoimmune disease.
Origin of International Alopecia Day
International Alopecia Day was initiated by American activist Lynn W. Walker in 2011. She herself lives with a diagnosis of alopecia totalis and created this day to unite people with similar experiences, reduce stigma, and highlight beauty and strength regardless of the presence of hair.
Theme of Alopecia Day 2025
This year's theme as per Alopecia UK is, 'Strength in Numbers', which urges more and more people to join the International Alopecia community and to do away with the shame of hair loss and form a support group, across the world.
Alopecia And Its Kinds
As per the National Library of Medicines, US, alopecia refers to the loss or absence of hair in areas where it normally grows. It can be localized or widespread, temporary or permanent, and affects people of all ages and genders. As a symptom with diverse underlying causes, alopecia is generally categorized into two main types: nonscarring (the most common) and scarring (cicatricial).
For many patients, hair loss leads to significant emotional distress and a reduced quality of life. Accurate diagnosis requires a thorough history, physical examination, and targeted investigations to identify the root cause and guide effective treatment. Managing alopecia can be challenging, but this overview outlines key assessment and treatment approaches for the most common forms to support better outcomes.
Forms of alopecia
There are several main types of alopecia, including:
alopecia areata: an autoimmune disease that causes hair loss, often in small, round patches on the scalp, but it can occur anywhere on the body
alopecia totalis: complete loss of scalp hair
alopecia universalis: hair loss over the entire body
androgenetic alopecia: hereditary baldness
Ways You Can Participate In International Alopecia Day
- Learn and share knowledge about alopecia
- Participate in activities for Alopecia Day
- Embrace the symbol by wearing blue
- Share you story to build a support group
- If you are a doctor, you can also advocate for the right alopecia treatment
- Contribute financially to alopecia research and awareness groups
- Volunteer to help spread alopecia awareness
- Raise voice against bullying, as many people are bullied due to their hair loss
© 2024 Bennett, Coleman & Company Limited

