- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
F.D.A. Approves New Non-hormonal Menopause Drug To Reduce Hot Flashes

Credits: Canva
Menopause is often seen as the end of womanhood by many women, however, as Dr Archana Nirula, a gynaecologist & obstetrician, who specializes on women’s health, says, "menopause is not the end of woman's life". Thanks to the new drug Lynkuet, finally approved by the US Food and Drug Administration, women can now actually believe so.
This is a nonhormonal medication, designed to treat hot flashes and night sweats in menopausal and perimenopausal women. The drug is developed by Bayer, and its main compound is elinzanetant. This is a novel therapy that could also help improve sleep disturbances commonly linked to menopause.
Why Is This Medicine A Breakthrough?
While most medicines treating menstrual-relating symptoms for women are heavy doses of hormones, leading to many side effects, Lynkuet stands out. It offers relief without using hormones, a much-needed alternative for women who cannot or prefer not to take hormone therapy.
What's Wrong With Hormonal Drugs?
while hormone replacement therapy (HRT), usually estrogen and progesterone, remain effective for many, it is not suitable for everyone. Women with a history or high risk of breast and ovarian cancer, or those wary of hormone-related side effects, often seek for better, safer and nonhormonal options.
Until recently, those options were extremely limited. The first nonhormonal drug, Veozah (fezolinetant), was approved in 2023, targeting only one type of brain receptor linked to temperature regulation. Before that, doctors mostly relied on low-dose antidepressants like paroxetine to manage hot flashes, an off-label approach with limited benefits.
How Does Lynkuet Work?
What makes this medicine different is the way it works on the brain. The drug is responsible for blocking two types of neurokinin receptors, namely: NK1 and NK3. These neurokinin receptors play a key role in regulating body temperature, mood, and sleep. By doing so, elinzanetant helps restore balance in the hypothalamus, which is brain's temperature control center. This usually becomes disrupted when estrogen levels fall during menopause.
This dual-action mechanism is what sets Lynkuet apart. Unlike Veozah, which targets only one receptor, elinzanetant’s two-receptor approach could mean broader benefits, not just reducing hot flashes and night sweats, but also improving sleep quality, which many women struggle with during perimenopause and menopause.
How Did It Pass The Clinical Trials?
Clinical trials for Lynkuet involved women aged 40 to 65 who experienced frequent and severe hot flashes. According to researchers, symptom relief began as early as one week after starting treatment.
By week 12, over 70% of women taking elinzanetant reported at least a 50% reduction in the frequency of hot flashes, compared to just over 40% in the placebo group. Sleep quality also improved significantly, with many participants reporting fewer disturbances at night.
At 26 weeks, the benefits persisted, more than 80% of participants maintained a 50% reduction in symptom frequency. Those who initially received the placebo and later switched to the drug showed similar improvements, underscoring its consistent efficacy.
Are There Any Side Effects?
The most common side effects reported are mild headache, fatigue, and joint pain. A few participants also reported elevated liver enzyme levels, however there was no serious toxicity observed.
New Mpox Strain Detected in California Sparks Health Alert
Credits: Canva
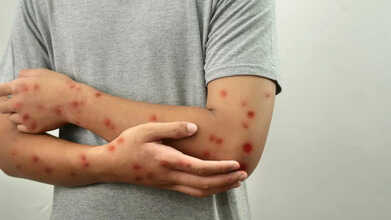
More than two years after the mpox outbreak in the United States was officially declared over, a fresh cluster of cases in California has caught the attention of infectious disease experts.
The previous U.S. outbreak, which spread primarily among men who have sex with men, was declared over in early 2023, though sporadic cases have continued to appear. Now, health officials are worried after identifying three unrelated mpox cases in California linked to a more infectious and potentially more severe strain of the virus known as clade I mpox.
What Is Mpox?
Mpox, previously called monkeypox, is a viral infection caused by the monkeypox virus, a member of the Orthopoxvirus genus. According to the World Health Organization, the virus is divided into two main groups—clade I (which includes Ia and Ib) and clade II (which includes IIa and IIb). The 2022–2023 global outbreak was linked to the clade IIb strain. The disease continues to pose a global health concern, with a sharp rise in cases reported in the Democratic Republic of the Congo and other regions due to clades Ia and Ib, and now in the United States.
How This Strain Differs From the 2022 Outbreak
The mpox variant responsible for the 2022 outbreak belonged to clade II, a strain with a relatively low fatality rate. During that outbreak, over 32,000 infections were reported nationwide, resulting in 58 confirmed deaths—a mortality rate of about 0.2 percent.
Clade I mpox, by comparison, has been found to cause more serious illness and spread more easily. Both strains exist in parts of Central Africa, but clade I has historically shown higher transmissibility and mortality than clade II.
California Cases Raise Alarm Over Possible Community Spread
All three of the California patients infected with the clade I strain required hospitalization. According to the California Department of Public Health (CDPH), the pattern of infection suggests that, much like the 2022 outbreak, transmission is again occurring primarily within networks of men who have sex with men.
Dr. Joseph Cherabie, assistant professor of infectious diseases at Washington University in St. Louis and a board member of the HIV Medicine Association, said it was “only a matter of time” before clade I reached the U.S.
While a previous California case of clade I mpox was linked to travel in Africa, these recent infections have no known travel connections, a detail that worries experts. “That makes us a little bit uncomfortable as epidemiologists and as public health folks,” Cherabie said, adding that it signals local transmission that health authorities may not yet be fully detecting.
Transmission and Concerns About Undetected Cases
Mpox spreads mainly through direct skin-to-skin contact with someone who is infected, often through the painful rashes characteristic of the disease. Since the source of infection for these cases remains unclear, Cherabie believes there are likely more undetected cases circulating in the community.
When mpox first appeared in nonendemic countries in 2022, health agencies faced the challenge of having no treatments or vaccines made specifically for it. However, existing smallpox treatments were found to be effective against mpox, given their viral similarities.
Treatment and Vaccine Options
The smallpox vaccine Jynneos and the antiviral drug tecovirimat (Tpoxx) continue to be the leading tools for protection and treatment. Experts expect them to work against both clade I and clade II mpox.Jynneos requires two doses for complete protection, while Tpoxx can be given either intravenously or orally, depending on the severity of symptoms.
Texas Sues Tylenol Makers After Trump Links Painkiller To Autism — All You Need to Know

Credits: Canva
Texas sues tylenol: Texas Attorney General Ken Paxton announced on October 28 that he has filed a lawsuit against the makers of Tylenol, accusing them of failing to warn consumers about possible risks of using the drug during pregnancy. According to USA Today, the lawsuit targets Johnson & Johnson and its spin-off company Kenvue, alleging they concealed information about Tylenol’s potential links to autism and attention deficit hyperactivity disorder (ADHD), violating state consumer protection laws.
“These corporations lied for decades, knowingly endangering millions to fill their own pockets,” Paxton said in a statement. “When they saw accountability coming, Johnson & Johnson tried to dodge responsibility by offloading their liability to another company. By holding Big Pharma accountable for poisoning our people, we will help Make America Healthy Again.”
This marks the first lawsuit by a state government since President Donald Trump recently claimed that using Tylenol during pregnancy may raise the risk of autism — a claim that has limited scientific support.
Tylenol: Why Is Texas Suing Tylenol Makers?
Texas’s lawsuit, the first of its kind from any state, follows remarks made last month by President Trump and Health and Human Services Secretary Robert F. Kennedy Jr., who jointly issued new guidance discouraging pregnant women from using acetaminophen, the active ingredient in Tylenol. They cited it as a possible factor behind autism, sparking widespread concern and confusion among expecting mothers and healthcare experts alike.
The research surrounding Tylenol’s use in pregnancy remains unsettled. While a few studies have suggested a possible link between prenatal exposure and autism, many others have found no such connection. Major medical organizations have since pushed back against Trump and Kennedy’s statements, warning that the claims could spread unnecessary fear and misinformation.
This lawsuit is the first to formally adopt Trump’s theory that acetaminophen use in pregnancy might cause developmental disorders in children. The issue has long circulated among Kennedy’s followers, but Trump’s public comments brought it into mainstream discussion.
Texas Sues Tylenol: Tylenol Maker Fights Autism Warning Label
Kenvue has strongly defended Tylenol’s safety and dismissed Trump’s allegations, calling them misleading. Johnson & Johnson has also maintained that it has always acted responsibly in its product labeling, warning users only about the proven risk of liver damage from overuse.
In an official response, Kenvue stated that it was “deeply concerned by the spread of misinformation about the safety of acetaminophen and how it may affect women and children.” The company emphasized that acetaminophen remains “the safest pain relief option for pregnant women when needed throughout pregnancy.” Without it, they argued, women could face dangerous choices, either enduring untreated pain and fever, which may harm both mother and baby, or turning to more hazardous alternatives.
Over the past few years, hundreds of lawsuits have been filed nationwide by parents who believe their children developed autism or ADHD after prenatal Tylenol exposure. The largest group of such cases, consolidated in federal court in New York, was dismissed earlier this year due to insufficient scientific evidence. Plaintiffs have appealed the ruling, with an appellate hearing scheduled for November 17.
Texas Sues Tylenol: Is Acetaminophen Linked to Neurodevelopmental Disorders?
According to The New York Times, scientists have explored possible links between acetaminophen use during pregnancy and developmental disorders for years, but the research remains inconclusive.
Medical associations have rejected the Trump administration’s recent warning, reiterating that Tylenol is still considered the safest pain reliever for pregnant women. They warn that avoiding it altogether could be dangerous, as untreated high fevers and severe pain can pose serious health risks to both the mother and the unborn child.
Is The White House Hiding Truth About President Donald Trump’s Health?

Credits: Canva
President Donald Trump confirmed on Monday that he recently underwent an MRI at the Walter Reed National Military Medical Center but chose not to explain the reason behind it. The statement marks the first time Trump, 79, has spoken about his second medical visit this year.
The October 10 appointment, described by the White House as a standard yearly exam, has stirred speculation since it came just six months after his last comprehensive physical. “I did. I got an MRI. It was perfect,” Trump told reporters on his flight to Tokyo. When asked for more information, he declined to say more, saying journalists should “ask the doctors.” This has left many wondering if there is more to the story behind his current health condition.
Is The White House Hiding The Truth About Donald Trump’s Health?
The White House has declined to clarify when Trump last took a cognitive test, as his most recent medical summary does not mention one. Officials have also refused to provide any additional information about the MRI that Trump said he underwent earlier this month. His admission has renewed speculation about the 79-year-old president’s health.
While Trump maintains that he is in “excellent shape,” the timing and secrecy surrounding the MRI have raised eyebrows. MRI scans are often used to detect issues such as strokes, brain abnormalities, or tumors. “I got an MRI. It was perfect,” Trump told reporters aboard Air Force One, but no further explanation has been provided.
White House Report Did Not Mention Trump’s MRI
Despite Trump’s assurance that his doctors had released a “very conclusive” report, the official White House summary did not specify why he made a second visit to Walter Reed within the same year. Traditionally, presidents undergo one full medical examination annually.
According to CNN, White House physician Dr. Sean Barbabella’s report only noted that Trump had “advanced imaging,” a term that could refer to CT scans or other procedures. There was no mention of an MRI or any details about its purpose or findings.
Another missing detail was any reference to a cognitive assessment, which had been included in Trump’s first examination earlier this year. Following his April check-up, Dr. Barbabella’s report had spanned three pages, covering Trump’s lab work, prescribed medication, vital signs, and overall physical condition.
Donald Trump’s Medical Tests
During the April examination, Trump’s doctor confirmed that the president had taken the Montreal Cognitive Assessment (MoCA), a 30-point test that screens for memory and attention issues. The report at the time noted, “A comprehensive neurological exam revealed no abnormalities in mental status, cranial nerves, motor and sensory function, reflexes, gait, or balance.” It added that Trump scored a perfect 30 out of 30 on the MoCA, showing no signs of cognitive decline.
By contrast, Trump’s latest medical update, released after his October visit, was a brief five-paragraph note with no mention of any cognitive evaluation. When reporters asked if the president had taken one, the White House did not respond.
Trump did, however, bring up cognitive tests during a speech on Monday, mocking Democratic congresswomen Alexandria Ocasio-Cortez and Jasmine Crockett as “low IQ” individuals who “wouldn’t do well” on such exams. Yet he stopped short of revealing when he last took the test or what his latest score might have been.
So, Is the White House Really Hiding the Truth About President Donald Trump’s Health?
Trump’s second visit to Walter Reed in six months, along with visible bruising on his hands and swelling in his ankles, has added to speculation about his condition. His acknowledgment of an MRI, which is absent from the official White House report has only intensified public curiosity. The lack of transparency surrounding the test and not mentioning details in the official statement have led many to question whether the White House is concealing information about the president’s health.
© 2024 Bennett, Coleman & Company Limited

