- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
FDA Approves Once-Monthly Injection For Hereditary Angioedema; What It Means For Long-Term Treatment?
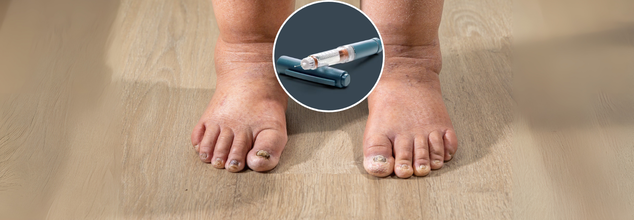
As a significant breakthrough in rare disease therapy, the U.S. Food and Drug Administration (FDA) has just approved Andembry (garadacimab‑gxii), the first-ever prophylactic treatment that aims for factor XIIa to prevent attacks of hereditary angioedema (HAE). This once-monthly injectable has provided patients 12 years and older with a new, convenient way to manage symptoms effectively—but its significance extends far beyond convenience, as it is a sea change in the way we treat this incapacitating illness.
Hereditary angioedema is an inherited, rare illness that results in sudden, aching swelling of the body's different regions, such as the face, limbs, or inner tissues like the airway or intestines. Blockages caused by it can result in life-threatening respiratory problems or severe abdominal pain. These occur because leakage from small blood vessels lets fluid seep into nearby tissues. HAE typically presents in childhood or adolescence, worsens after puberty, and strikes almost everyone diagnosed through age 20.
While the swelling may be painful and disfiguring, the most significant danger is airway involvement, which can be fatal. Conventional treatments are primarily concerned with treating these sudden attacks or maintaining regular infusions to prevent them. But until now, real long-term prevention was out of reach.
How Andembry Works?
Andembry is a monoclonal antibody that directly blocks factor XIIa, a central protein in the cascade of processes that leads to HAE attacks. By blocking factor XIIa's action, Andembry stops the process before swelling occurs—making the first line of defense against unpredictable flare-ups.
Administered through a straightforward autoinjector that delivers the dose in 15 seconds or less, Andembry is designed to become an integral part of patients' lives. Users simply have to self-administer the injection on a monthly basis, making it a convenient option for teenagers as well as adults who are treating this condition.
The FDA's approval is based on powerful Phase III clinical trial data. During six months, 62% of Andembry patients were wholly attack-free—a remarkable benchmark for a disease characterized by constant flare-ups.
Are There Any Possible Side Effects?
Like any drug, Andembry has potential side effects, although most were mild. The most frequent problems encountered involved nasal and upper throat inflammation and abdominal pain, each of which were observed in approximately 7% of patients. Most importantly, no severe adverse events or drop-outs from treatment were seen—testifying to the drug's overall safety and tolerability.
Such a positive profile represents an important victory for a long-term treatment—particularly one that will be repeatedly self-administered by families and patients.
Dr. Bill Mezzanotte, R&D Head at CSL (who created Andembry), refers to the drug as a "game-changer". He mentions it's the first monoclonal antibody to be created wholly by CSL and the only injectable treatment for factor XIIa. The fact that it's designed to give treatment in seconds speaks to a patient-centric focus—particularly necessary for a disease that is notoriously unpredictable and can hit at any time.
What This Means for HAE Treatment and Care
Apart from clinical efficacy, Andembry vows to greatly improve the daily lives of HAE patients. The benefits in everyday life are:
- Relief from emotional distress of never knowing when the swelling will occur
- Fewer visits to emergency rooms and reliance on on-demand drugs
- More independence to travel, socialize, and enjoy activities
- Better school or work productivity because of more consistent health
Preventing attacks before they occur can cut down school or workplace absences, decrease hospital visits, and reduce emergency care expenses—all substantial quality-of-life differences.
Andembry's approval represents a tipping point in the direction of personalized, preventive treatment for HAE. It changes the emphasis from responding to swelling attacks to actively preventing them, a shift with broad implications for patients and the health care system as a whole.
Providers now have a powerful, easy-to-use option to present to patients and families. Investigators are looking at whether Andembry can supplant or minimize the use of current prophylactics, like C1 inhibitor treatments or bradykinin receptor antagonists.
Economically, although monoclonal antibody therapies are generally very expensive, Andembry's ability to decrease emergency room visits and hospital stays could potentially yield dramatic cost savings for both patients and payers over time.
With Andembry's approval, individuals with hereditary angioedema can now receive a once-per-month injection that provides near-total attack prevention, outstanding safety, and improved quality of life. For the increasingly large population of diagnosed and undiagnosed patients, Andembry provides a new level of confidence and control in managing a condition long known to be capricious and highly restrictive.
Becky Quick Speaks About Her Daughter’s Genetic Disorder —What The Diagnosis Is About

Credits: AP
Becky Quick recently spoke about her 9-year-old daughter Kaylie’s private struggle with a rare genetic disorder. In an interview with People, published Thursday, January 8, the 53-year-old co-anchor of CNBC’s Squawk Box revealed that Kaylie has SYNGAP1. According to the Child Neurology Foundation, SYNGAP1 is a genetic condition that can cause seizures and developmental challenges. Quick recalled that she first noticed something was off “probably around 8 months,” when Kaylie wasn’t reaching her developmental milestones.
“Sometimes her eyes would cross. You just know as a mom that something isn’t right,” Quick told People, adding that after observing her daughter and reviewing developmental studies, it became clear that Kaylie wasn’t meeting expected benchmarks. “We immediately began working with therapists. They supported Kaylie in rolling over, achieving fluid movements, eventually walking, and so many other skills. But we also noticed additional concerns developing.”
Becky Quick Daughter Battles SYNGAP1
For nearly a decade, Quick has kept her daughter Kaylie’s SYNGAP1 diagnosis private. The condition, as defined by the Children’s Hospital of Philadelphia, is a rare genetic disorder that can lead to a variety of neurodevelopmental challenges. It currently has no cure and often requires round-the-clock care.
The journalist took Kaylie to her pediatrician, who recommended a study with a developmental specialist. The results confirmed Quick’s fears: her daughter was not reaching key milestones.
“We started working with therapists immediately,” Quick said. “[They] helped Kaylie develop rolling, fluid movement, eventually walking, and many other skills. But we also observed additional issues.”
A neurologist then performed an EEG study, which revealed that Kaylie was experiencing subclinical seizures — subtle seizures that aren’t visibly noticeable. A subsequent genetic test confirmed that Kaylie had SYNGAP1.
What Is SYNGAP1?
SYNGAP1 (SRD) refers both to a gene and the rare neurological disorder it causes. The condition is marked by intellectual disability, epilepsy, and autism spectrum traits, arising from a mutation that reduces the SynGAP protein, which is essential for proper synaptic function in the brain, according to MedlinePlus. Without adequate SYNGAP1 protein, synapses become overly excitable, impairing communication between neurons and leading to the neurological difficulties observed in SYNGAP1 patients, as per National Institute of Health.
Becky Quick Daughter: What Are The Symptoms Of SRD?
SYNGAP1 is considered a spectrum disorder, meaning patients experience symptoms differently and with varying severity. The exact factors influencing symptom expression or intensity remain unclear. The most commonly observed signs include, as per NIH:
- Intellectual Disability (mild to severe)
- Hypotonia (low muscle tone)
- Global Developmental Delay
- Epilepsy (including subtle eyelid flutters, brief jerks, staring spells, and drop seizures)
- Sensory Processing Disorder
- Delays in Gross and Fine Motor Skills
- Dyspraxia (coordination difficulties)
- Speech Delay/Apraxia (mild to severe)
- Autism Spectrum Disorder
- Sleep and Behavior Disorders
- Visual Abnormalities
- Not every patient will exhibit all of these symptoms.
Becky Quick Daughter: What Causes SRD?
SYNGAP1-related disorders arise from a variant on the SYNGAP1 gene (6p.21.32). Each human cell contains 23 pairs of chromosomes, totaling 46, with thousands of genes on each chromosome. Most genes come in pairs, one inherited from each parent, and their primary role is to produce proteins that regulate tissues and organs.
When a gene experiences a variant, sometimes called a mutation or alteration — it can stop functioning properly. Variants occur naturally, similar to a typo when DNA is copied from cell to cell, or they may result from environmental factors.
A de novo variant is a genetic change that appears for the first time in a family, occurring early in reproductive development. Most SYNGAP1 patients carry de novo variants. For families with a genetic report, understanding the variant can help clarify the diagnosis and guide next steps.
India’s Antacid Habit Is Putting Hearts, Kidneys, Guts, and Bones at Risk

Credits: iStock
Indians are popping antacids like candies, and they are putting their hearts, kidneys and gut health at risk. Doctors from time and again have cautioned patients to not take such pills without prescriptions.
Antacids are prescribed proton pump inhibitors or PPIs, used commonly to reduce the amount of acid in the stomach and helps to treat and prevent various acid related conditions. However, in India, acidity is not treat as a symptom, but a lifestyle condition, which has made these pills so common. These over the counter access also created medical complacency, this means the availability has blurred the line between short term relief and long term therapy.
How does antacid impact your kidney health?
Repeated use is increasingly associated with acute interstitial nephritis and chronic kidney disease. What makes this dangerous is that kidney damage often develops silently, discovered only when kidney function has already deteriorated significantly.
How does antacid impact your gut health?
It also impact the gut microbiome, and causes chronic digestive problems. Stomach acid regulates gut bacteria. Suppressing it allows harmful bacteria to flourish, leading to bloating, infections, diarrhea, and conditions like small intestinal bacterial overgrowth (SIBO).
How does antacid impact your bone health?
Furthermore, regular usage of antacids could make your bones weak too. As per a 2023 study published in the journal BioMed Research International, pantoprazole cause bone loss, which could be prevented by adding octreotide.
The study analyzed the serum levels of calcium, phosphorus, and ALP before starting the treatment, and at the end of 12 weeks of treatment on pantoprazole, significant decline in calcium levels were noticed, as compared with other groups. The study also found that octreotide significantly prevented the effect of pantoprazole on the serum levels of calcium and ALP.
The study also found that pantoprazole decreased femoral bone density and femoral BMAD. Besides this, another decrease was found in the femoral bone weight and volume as well as the trabecular volume.
How does antacid impact your heart health?
Frequent heartburn is also a symptom of gastroesophageal reflux disease or GERD. This is a condition in which the valve between the stomach and the lower esophagus malfunctions and allows stomach acid to bubble up into the esophagus. This is often treated with antacid, which may mask the real problem.
Over time, untreated GERD can injure the lining of the esophagus and increase the risk of serious complications, including Barrett’s esophagus, a condition that can raise cancer risk. Also, symptoms often blamed on heartburn, such as chest pain or burning, can sometimes signal a heart attack. That’s why experts stress getting a proper medical evaluation before self-treating with antacids.
The safer alternatives are:
Famotidine (Pepcid, Calmicid, Fluxid, Mylanta AR) is a potent H2 blocker used to manage acidity and heartburn. Studies show that famotidine is not thought to raise the risk of osteoporosis.
Other options: Ranitidine (Zantac - where available, as it was withdrawn in some markets due to safety concerns) and Nizatidine are other H2 blockers.
Note: Health & Me do not encourage discontinuance of any prescribed medicine by a doctor. Before making any change in your medicine schedule, please speak to your doctor/GP.
Nasal Spray Warning Over 'Rebound Congestion'; Experts Say It Should Not Be Used For More Than 7 Days

Credits: iStock
Nasal spray warnings are given by doctors and experts as the long use of it could lead to worsening of condition. The Royal Pharmaceutical Society (RPS) advised the public to not use nasal decongestant for more than seven days, as it contains xylometazoline or oxymetazoline. Its prolonged use can cause 'rebound congestion' or increased dependency on these sprays to breathe easily.
A recent poll, reports the Independent found out that almost six in 10 pharmacists report patients were unaware of the dangers of its extended use. Due to the increased number of flu activity, symptoms like blocked nose has increased, which has lead to a high usage of nasal spray.
Is Rebound Congestion Preventable?
It is a preventable condition, and is scientifically known as rhinitis medicamentosa, which causes the symptoms to worsen. Patients become depended on the sprays to breathe more easily.
RPS survey of 300 pharmacists found that 59% think the public is not aware of the risks, while 75% said packaging should be clearer about the seven-day limit. 63% said they had intervened in cases of suspected overuse.
Professor Amira Guirguis, chief scientist at RPS told the ITV News, "Nasal decongestant sprays can be helpful for short-term relief, but using them for longer than seven days can make your congestion significantly worse. Our research shows that many people are unaware of this risk, which means they may continue using these sprays without realizing they could be prolonging their symptoms. We'd like to see clearer warnings on the packaging which you can't miss and greater awareness of the seven-day limit. If your congestion lasts more than a week, speak to your pharmacist. There are safe and effective alternative options to help you manage your symptoms."
Another survey by ITV News suggests that more than a fifth of adults have used the products for longer than seven days. This means 5.5 million people in the UK may have risked developing a dependency.
Read: Bristol Hospitals Under Severe Strain as Flu and Cold Weather Hit the Region
As ITV News reported, Charlotte Johnstone, who is 30, had been using nasal spray multiple times a day since she was seven years old. She realized that the impact of this so-called addiction has caused her anxiety and left her "dreaming about not being able to breathe". She told ITV News, “I can’t sleep without having it, I wake up and the first thing I do is have my nasal spray. I don’t like eating if I’ve got a blocked up nose, it just makes me feel claustrophobic. I wouldn’t put myself in a situation where I don’t have it. “I go through stages of losing my sense of smell. I know it’s doing something but I don’t know what. But for the sake of having a clear nose, I’ll just take it,” she said.
A spokesperson from PAGB, the consumer healthcare association representing the manufacturers of these products, said: “The patient information leaflet which accompanies all nasal decongestant sprays, includes these instructions and outlines the risks of taking the medication for longer than its indicated use. As explained on the information supplied with the nasal decongestant sprays, OTC medicines manufacturers provide comprehensive accessible information to support people to make responsible informed decisions about the right product to self-care for their self-treatable condition," reported ITV News.
© 2024 Bennett, Coleman & Company Limited

