- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
FDA Plans To Halt Animal Testing For Drug Approval, What Could Replace It?

Credits: Canva
In a significant shift, the US Food and Drug Administration (FDA) has announced its plan to reduce its reliance on animal testing for drug development. This is especially for monoclonal antibody therapies and other advanced medicines. The agency now plans to use more human-relevant technologies in order to improve safety, lower costs and also speed up the approval process.
Animal History Traces Back To Ages
Animal testing has remained the cornerstone of all experiments, whether medicine or space. Since the 1930s, when the US passed the Federal Food, Drug, and Cosmetic Act in response to the tragic consequence of untested medication, animal testing was mandated. Scientists have been using mice, rats, rabbits and other animals to test for toxicity, side effects, as well ass efficacy before any new drug could be forwarded for human trial.
Over the decades, this practice also helped introduce many life-saving treatments. However, it has also raised ethical concerns and scientific questions, especially on how accurately can animal models really predict human response.
Why Is FDA Now Moving Away From Animal Testing?
The FDA’s new initiative represents a "paradigm shift," according to its Commissioner, Dr. Martin A. Makary. There are several reasons behind this change:
Limited Predictive Value: Drugs that work safely in animals don’t always perform the same way in humans. This mismatch can lead to failed clinical trials or unexpected side effects in people.
Ethical Concerns: As public awareness and concern about animal welfare have grown, so too has the demand for more humane research methods.
Cost and Time: Animal testing is expensive and time-consuming. Each stage of testing can take months or years and cost millions of dollars.
Scientific Advancements: New technologies now offer better ways to model human biology and disease. These methods not only spare animals but also yield more accurate data.
What Can Replace Animal Testing?
The FDA is planning to incorporate innovative tools like computer modeling and AI, human organoids, and organ-on-a-chip technology, as well as real world human data.
How Will these Work? The computer modeling and AI will allow the scientists with the help of simulations to predict how a drug will behave in the human body based on its chemical structure, genetics, and existing medical data.
Furthermore, human organoids are miniature, lab-grown versions of human organs like a liver or brain which are made from stem cells. They also closely mimic how real organs function and could be used to test drug safety and effectiveness.
Organ on a chip technology involves tiny chips that simulate the activity of the entire organ systems. For instance, how the heart beats or the lungs breathe. It allows for more accurate and efficient testing.
The agency is also planning to rely more on existing human clinical data from other countries with comparable regulatory standards. If a drug has already been tested and used abroad, repeating animal testing for the same in the US should not be any longer a mandate.
The new approach will be applied immediately to investigational new drug applications—essentially, the first step in bringing a new treatment to the U.S. market. This means that pharmaceutical companies can now submit non-animal safety data as part of their applications.
Importantly, this doesn’t mean animal testing will be banned entirely. In some cases, it may still be required to answer specific safety questions. However, the goal is to make animal studies the exception, not the rule.
Nipah Virus Survivor Speaks Out, Calls for More Awareness

Credit: Canva
The Nipah virus outbreak began in West Bengal, India with two hospital nurses at AIIMS, Kolkata, testing positive for the infection and being quarantined, prompting widespread testing. Soon after, five cases, including a doctor and a staff member, were confirmed and over 100 people were quarantined.
However, one of the nurses, a 25-year-old unidentified man has now made a recovery and revealed his experience with the virus, claiming that despite irritation in the throat and uncertainty about what lay ahead, he had faith in his doctors and fellow nurses.
In an interview with the Metro, he said: “After I was taken off ventilation and regained consciousness, I came to know that I have Nipah. I still had the tube in my mouth, and there was irritation. Despite the irritation and my fear, I had faith in the doctors and nurses.
“I have suffered and I know the symptoms. I will tell people when they should get checked for the Nipah virus. I want to raise awareness about the virus and its symptoms.
“I am not sure how I came in contact with the deadly virus. Maybe it was while treating a patient. But I will continue to work as a nurse. I am waiting to rejoin the hospital,” he added.
The unidentified healthcare professional remains very weak physically and is undergoing physiotherapy to regain his strength. “I was bedridden for over a month. I am still very weak and have an unstable gait. So, I am undergoing physiotherapy,” he said.
The other nurse, a woman, remains in a coma but has been taken off ventilation support, a hospital official confirmed this week. .
Nipah Virus: What Is It And What Are Its Symptoms?
According to WHO, Nipah virus is a zoonotic illness which means it is mostly transmitted from animals to humans through bats. However, it can also spread through fruits that have been contaminated by the saliva, urine or droppings of infected bats. Human-to-human transmission can also occur through close contact with an infected person or their bodily fluids.
The illness has a 75 percent fatality rate and there are no vaccines to protect the public.
The virus was first identified in 1998 during an outbreak among pig farmers in Malaysia and soon made its way to India and Bangladesh in 2001 with cases often involving family members or caregivers tending to infected patient.
READ MORE: Nipah Virus Outbreak In India: Myanmar Airport Tightens Health Screenings
Although Nipah virus has caused only a few known outbreaks in Asia, it infects a wide range of animals and causes severe disease and death in people. Some of its common symptoms include:
- Fever
- Headache
- Breathing difficulties
- Cough and sore throat
- Diarrhea
- Vomiting
- Muscle pain and severe weakness
Samples collected from the patient’s home and workplaces, including pets and partially eaten fruits dropped by bats, all tested negative for the virus, and the exact source of the infection could not be identified.
What Does WHO Say?
The World Health Organization has declared it is unlikely India's deadly Nipah Virus outbreak will cross borders and reach other nations, noting that countries do not need to set any travel restrictions in place.
In an email to Reuters, officials said: "The WHO considers the risk of further spread of infection from these two cases is low". adding that India has the capacity to contain such outbreaks.
"There is no evidence yet of increased human to human transmission," it said, adding that it has coordinated with Indian health authorities.
While health officials state it is nearly impossible for the virus to transmit across countries and unlikely to cause an international outbreak, countries including Australia, Vietnam, Thailand, Malaysia and Singapore continue to remain on high alert and have begun airport screenings.
Ahmedabad Toddler Swallows Hulk Toy, Showed X-Ray, Doctors Remove It Via Endoscopy
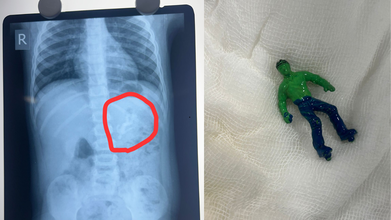
Credits: X
Ahmedabad toddler, one-and-a-half-year-old boy swallowed a 'Hulk' toy, which is based on a popular comic superhero. The toy was stuck in his stomach when his parents took him to the Civil Hospital. According to reports by News18, the child is identified as Vansh who showed the signs of discomfort and began vomiting. This is what alarmed the parents.
As per the News18 report, his mother Bhavika was suspicious when she noticed that one of his toys was missing. The child was rushed to the hospital and an X-ray revealed that he had swallowed the entire plastic toy. The toy was not broken.
Hindustan Times reported that Dr Rakesh Joshi, Head of the Department of Pediatric Surgery removed the toy through upper GI endoscopy. "Had it been a little late, the toy could have moved further from the stomach and got stuck in the intestines. In that case, there would have been a risk of intestinal blockage and even rupture," the senior doctor said.
"There is a natural valve between the esophagus and the stomach. The biggest challenge was to take out a whole toy through this valve. When we tried to grab it with the endoscope, the toy kept slipping because of the air in the stomach. Pulling the toy by its hand or foot raised the possibility of it getting stuck in the valve and causing it permanent damage," he said.
The doctor noted that if the toy had further slipped down, it would have increased the risk of intestine rupturing.
Ahmedabad Toddler Swallows Hulk Toy: What Parents Must Keep In Their Mind
Under the Toys (Quality Control) Order, 2020 issued by the Department for Promotion of Industry and Internal Trade under the Ministry of Commerce and Industry, toy safety in India was brought under mandatory BIS certification from September 1, 2020. The move aims to ensure safer toys for children while also supporting the government’s policy of curbing non-essential imports.
Industry sources estimate that more than 85 percent of toys sold in India are imported. Officials say the Toys Quality Control Order is a key step in preventing the entry of cheap and substandard toys into the domestic market, many of which fail to meet basic safety requirements.
Ahmedabad Toddler Swallows Hulk Toy: Standards for Electric and Non-Electric Toys
The quality control order clearly defines safety standards based on the type of toy. Non-electric toys such as dolls, rattles, puzzles, and board games must comply with IS 9873 (Part 1):2019. These toys do not rely on electricity for any of their functions.
Electric toys, which include at least one function powered by electricity, are required to meet the standards outlined under IS 15644:2006. Compliance with these standards is mandatory before such toys can be sold in the Indian market.
Ahmedabad Toddler Swallows Hulk Toy: Risks Linked to Untested Toys
Toys that are not tested by NABL-accredited toy testing laboratories can pose serious health risks to children. Sharp edges and poorly finished parts can cause cuts and injuries. PVC toys may contain phthalates, which are considered harmful chemicals.
Many low-quality toys have also been found to contain lead, a substance known to be particularly damaging to brain development in children. Soft toys with fur or hair can trigger allergies or become choking hazards. In some cases, small body parts can get stuck in gaps or holes, increasing the risk of injury.
Testing by NABL-accredited laboratories ensures that toys are safe, durable, and suitable for specific age groups. Parents are advised to check for IS marks on toys before purchasing, as this indicates compliance with Indian safety standards.
Ahmedabad Toddler Swallows Hulk Toy: What Parents Should Check Before Buying Toys
Experts recommend avoiding toys with small detachable parts for toddlers and young children, as they are more likely to put objects in their mouths. Toys should always match the child’s age, skill level, and interests.
Parents are also urged to look for IS marks, which confirm that the toy has been tested and certified. Loud toys should be avoided, as prolonged exposure to sounds above 85 decibels can harm a child’s hearing.
Electric toys with heating elements should be used with caution or avoided altogether due to burn risks. Finally, toys with sharp edges or shooting components should be carefully examined to prevent cuts and injuries.
Nipah Virus Outbreak In India: Myanmar Airport Tightens Health Screenings

Credits: iStock
Nipah virus outbreak in India triggered airport screenings of travelers, including in Myanmar. Many reports claim that passengers are being checked in similar ways as they were during the COVID-19 virus spread. Health and Me reported how in Thailand the health screenings of foreign travelers were taken seriously, a similar case is seen in Myanmar.
Nipah Virus Outbreak In India: How Are Travelers Screened In Myanmar?
Myanmar has tightened its health screenings and surveillance at Yangon International Airport to prevent any possible entry of Nipah virus case, reported The Global New Light of Myanmar. Travelers who are arriving from India, especially West Bengal are given special attention to check for any fever or other Nipah-related symptoms, read the report by the Ministry of Health.
The ministry also noted that health screening of passengers arriving from abroad is being conducted in line with the established guidelines for infectious diseases that could give rise to public health emergencies, Xinhua news agency reported.
Informational leaflets too are being distributed among travelers to be aware of the symptoms. Posters are also displayed at the airport. Along with all that, disease prevention and control measures are also being carried out in the airport.
Screening measures are also enhanced and implemented at Mandalay International Airport.
Nipah Virus Outbreak In India: What Is It?
As per the World Health Organization (WHO), Nipah virus infection is a zoonotic illness that is transmitted to people from animals, and can also be transmitted through contaminated food or directly from person to person.
In infected people, it causes a range of illnesses from asymptomatic (subclinical) infection to acute respiratory illness and fatal encephalitis. The virus can also cause severe disease in animals such as pigs, resulting in significant economic losses for farmers.
Although Nipah virus has caused only a few known outbreaks in Asia, it infects a wide range of animals and causes severe disease and death in people.
Nipah virus is infectious and can spread from animals like bats and pigs to humans through bodily fluids or contaminated food. It can also pass between people through close contact, especially in caregiving settings. While it can spread via respiratory droplets in enclosed spaces, it is not considered highly airborne and usually requires close, prolonged contact for transmission. Common routes include direct exposure to infected animals or their fluids, consuming contaminated fruits or date palm sap, and contact with bodily fluids such as saliva, urine, or blood from an infected person.
Nipah Virus Outbreak In India: What Are The Common Symptoms?
- Fever
- Headache
- Breathing difficulties
- Cough and sore throat
- Diarrhea
- Vomiting
- Muscle pain and severe weakness
© 2024 Bennett, Coleman & Company Limited

