- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Flu Shot Linked To Higher Risk Of Infection This Year, Study Raises Eyebrows- Should You Be Concerned?
Credits: Canva
This flu season has turned out to be quite a surprise. A new study from Cleveland Clinic indicates the 2024–2025 flu shot might have fallen short — and potentially worsened the situation. With vaccinated patients reportedly having higher infection rates, health professionals and the general public are now reassessing the vaccine's effectiveness in real life.
New research from the Cleveland Clinic has caused alarm among health professionals and the general public. In contrast to years of conventional wisdom, the current flu shot may not only have been useless — it could actually have made some people more susceptible to the flu.
Released as a preprint on MedRxiv.org, the Cleveland Clinic study compared infection rates among more than 53,000 of its own staff and concluded that vaccinated staff had a higher rate of flu infections than their unvaccinated peers. Although the study is not yet subject to peer review, early conclusions have sparked a firestorm of questions about vaccine effectiveness, public health policy, and efforts to prevent future influenza.
Carried out across 25 weeks of the 2024-2025 respiratory viral season, the study monitored flu infection rates among Cleveland Clinic staff — predominantly a cohort of healthy adults of working age.
- Vaccinated staff had a 27% higher rate of flu infections than the unvaccinated cohort.
- Effectiveness of the vaccine was estimated at -26.9%, reflecting not only diminished protection, but a seeming increase in risk.
- The rate of influenza initially tracked similarly in both groups, but increased more steeply in the vaccinated as the season went on.
Notably, almost 99% of the vaccinated workers got the trivalent inactivated influenza vaccine, so it is difficult to know whether other formulations of the vaccine might have produced different results.
What Does "Negative Vaccine Effectiveness" Really Mean?
Negative vaccine effectiveness — particularly in the range of -26.9% — is a dramatic and disconcerting statistic. Yet, it does not indicate the vaccine induces the flu. Rather, it suggests that in this specific season, the vaccine provided no significant protection and potentially was not well matched to the prevailing virus strains.
The Cleveland Clinic explained in a statement to Fox News Digital, "The results do not suggest that vaccination increases the risk of flu. Instead, the study implies that the effectiveness of this season's vaccine in preventing influenza may have been limited in relatively healthy healthcare workers."
That is to say, the virus could have caught up with the shot.
While the results are surprising, specialists point out that this study is far from being the last word regarding the effectiveness of flu shots. There were several limitations the authors have acknowledged:
The research did not test flu severity of symptoms, hospitalizations, or fatalities — which are important indicators of vaccine worth.
It tested only healthcare workers, but not children, elderly people, and immunocompromised individuals, who most usually benefit from flu vaccination.
The vast majority of participants were given one type of vaccine, so the performance of others remains unknown.
Dependence on home test kits could have resulted in underreporting or misclassification of certain cases.
Why the Flu Shot Works Differently Each Year?
Performance of flu vaccines differs from year to year because of a number of factors:
Strain Mismatch: Every year, vaccine manufacturers make an educated estimate of the most common flu strains. If they're incorrect, the vaccine will provide suboptimal protection.
Population Immunity: Immune response varies with age, health, and past experience with flu viruses or vaccines.
Mutation Rates: Flu viruses change rapidly. Even when production starts, strains can change, lowering efficacy.
This year could just have been an anomaly in terms of immune response or strain drift — but the stakes are high for how we handle flu vaccination in the future.
Physicians warn not to make quick judgments based on a single preprint study. Dr. [Name], infectious disease physician at [Institution], cautions:
While Cleveland Clinic research is definitely worth further examination, it's important to keep in mind that the flu vaccine's greatest aim is to curb severe illness and mortality. We just don't have enough data yet to realize the total effect of this year's vaccine.
The Centers for Disease Control and Prevention (CDC) still encourages yearly flu vaccination for all people six months and older based on long-standing proof that flu vaccinations decrease the possibility of serious complications from the flu.
Should You Still Get the Flu Shot?
The quick answer: Yes — but with awareness.
This study points out that flu vaccine effectiveness can vary, and in extreme cases, may even not protect as expected. But overall evidence still continues to affirm the vaccine's function of reducing hospitalizations, decreasing duration of illness, and safeguarding high-risk groups.
If anything, the results emphasize the importance of ongoing investment in the development of vaccines, improved real-time surveillance of viral mutations, and open reporting of vaccine performance.
The Cleveland Clinic study doesn't mark the end of the flu shot — but it does pose important questions about how we produce, test, and present vaccine effectiveness. As more evidence comes in and peer-reviewed evaluation is finalized, health authorities will most likely fine-tune their approach to prevent future flu seasons from catching us unprepared.
American Kids Have Become Unhealthier In The Past Two Decades

Credits: Canva
A new study has found that the overall health of children in the United States has declined over the past 17 years. The reasons? Rising obesity rates to increased mental health issues like depression and anxiety, American kids today are grappling with a broader range of health concerns than ever before.
How Was The Study Conducted?
The research, published in the Journal of the American Medical Association (JAMA) on Monday, was led by Dr. Christopher Forrest of the Children’s Hospital of Philadelphia. The study is one of the most comprehensive examinations of children's health in the U.S. to date. Using data from eight different sources—including national surveys, electronic health records from 10 pediatric systems, and international mortality statistics—the researchers analyzed 170 indicators of child health.
The findings offer a sobering overview, painting a clear picture of a consistent decline in physical and mental well-being among American children from 2007 to 2023.
What Did The Study Find?
Here are some of the most concerning findings from the study:
Obesity on the Rise: The percentage of children aged 2 to 19 who are obese jumped from 17% in 2007-08 to about 21% by 2021-23.
More Chronic Illnesses: Kids in 2023 were 15% to 20% more likely than kids in 2011 to be diagnosed with chronic conditions like anxiety, depression, or sleep apnea.
General Health Decline: The number of children affected by at least one of 97 chronic conditions increased from 40% to 46% over the study period.
Mental Health Red Flags: Reports of depressive symptoms, early puberty, loneliness, and trouble sleeping all saw significant increases.
Higher Death Risk: American kids were about 1.8 times more likely to die than their peers in other high-income nations. Infants born prematurely or those who experienced sudden unexpected deaths were more common in the U.S., and older kids faced a greater risk of dying due to firearms and car crashes.
The Study Results Are A Health Warning
“This isn’t just about one health issue—it’s about all of them moving in the wrong direction,” said Dr. Forrest. “The real surprise wasn’t in any single data point, but in how universally the data shows kids' health getting worse.”
The researchers believe these troubling trends are a reflection of larger problems within American society—ranging from poor nutrition and lack of exercise to increased screen time and systemic barriers to healthcare.
“Children are the canaries in the coal mine,” Forrest explained. “When their health changes, it signals deeper societal issues.”
National Policy in Question
Earlier this year, Health Secretary Robert F. Kennedy Jr. released the “Make America Healthy Again” (MAHA) report, warning that children in the U.S. are “undernourished and overmedicated.” While this report has brought much-needed attention to the topic, experts say that current government policies might be doing more harm than good.
Dr. Frederick Rivara, a pediatrician and researcher at Seattle Children’s Hospital, co-authored an editorial published alongside the study. “The health of kids in America is not as good as it should be and definitely not on par with other countries,” he said. “And the current policies of this administration are likely to make it worse.”
According to the editorial, the MAHA campaign’s focus on chronic illness is undercut by policies that eliminate injury prevention programs, cut maternal health services, reduce funding for infant death prevention, and promote vaccine hesitancy.
A Call for Community-Led Change
Forrest believes that addressing the problem will require more than national policies—it needs a ground-up approach. “We need to examine the environment children are growing up in, starting at the neighborhood level,” he said. “Let’s think of children’s health the way we think of ecological sustainability. If the ecosystem is unhealthy, so are the kids.”
Though the study does have limitations and may not fully represent every demographic across the U.S., experts like Dr. James Perrin of the American Academy of Pediatrics agree: “The basic finding is true,” he said. “Children’s health in America is getting worse—and we need to act.”
This Very Painful Symptom Is A Sign You’ve Got The New 'Highly Contagious' COVID Variant Stratus

Credits: Health and me
Just when the world had begun to settle into post-pandemic normalcy, a new COVID-19 variant has entered the spotlight—Stratus, a name now making headlines across the UK. If you haven’t heard of it yet, you will. Stratus, specifically its XFG and XFG.3 sub-variants, is spreading quickly and has prompted health experts to take notice.
There’s no reason to panic. The variant isn’t known to cause more severe illness or to evade current vaccines. But it is worth understanding, especially as COVID continues to evolve and resurface in new forms. The story of Stratus offers insight not just into the virus itself, but how we’ve learned to live alongside it—through better surveillance, faster response, and smarter precautions.
What Is the Stratus Variant?
Stratus is not a completely new virus—it’s a descendant of the Omicron lineage, already known for its ability to spread easily while generally causing milder illness than earlier strains like Delta.
More specifically, Stratus is what experts call a “recombinant” strain, formed when someone is infected with two variants at once and those variants mix their genetic material. This gives Stratus its nickname: the “Frankenstein” variant. There are two types of Stratus in circulation:
XFG
XFG.3, a spin-off which currently accounts for about 30% of COVID-19 cases in England, up from just 10% in May, according to the UK Health Security Agency (UKHSA).
Because of its rapid spread, the World Health Organization (WHO) has classified XFG as a "variant under monitoring", urging global surveillance.
What Makes Stratus Variant Different From Other COVID Strains?
Though most symptoms of COVID haven’t changed much with each variant, Stratus may come with a new, unusual symptom: a hoarse voice.
According to some UK-based clinicians, patients infected with XFG and XFG.3 have reported hoarseness more frequently. This could be a result of the strain’s impact on the upper respiratory tract, particularly the vocal cords but here’s the thing: this is not yet a definitive diagnostic marker. Hoarseness alone doesn’t confirm infection, and not everyone with Stratus reports it. Like other variants, symptoms remain largely dependent on the person’s immune response, vaccination status, and pre-existing conditions.
What Are the Unique COVID Symptoms of Stratus?
While Stratus may bring hoarseness into the conversation, the core symptoms of COVID remain largely the same. According to the NHS and UKHSA, people infected with Stratus may experience:
- High temperature or chills
- Persistent cough
- Shortness of breath
- Loss or change in taste or smell
- Fatigue
- Headaches
- Muscle aches
- Sore throat
- Nasal congestion or runny nose
- Loss of appetite
- Diarrhea or nausea
These symptoms also overlap with seasonal flu and allergies, making it difficult to identify the variant without testing. What makes Stratus stand out so far is its hoarseness, which might be more pronounced than in previous infections.
Is Stratus COVID Variant More Dangerous?
The WHO and UKHSA have both made it clear: there’s no current evidence that Stratus leads to more severe illness, higher hospitalization rates, or death compared to other Omicron subvariants.
Dr. Alex Allen, a consultant epidemiologist at UKHSA, explained that there is no indication vaccines are less effective against Stratus. In fact, immunity—whether from vaccines, previous infection, or both—continues to offer protection, especially against serious outcomes.
It’s also worth noting that hospital admissions and case rates in the UK have been trending downward, despite the rise of Stratus. As of late June, COVID hospitalizations in England dropped from 1.46 per 100,000 to 0.99 per 100,000.
This suggests that while XFG and XFG.3 are spreading fast, they may not be driving a significant wave of severe disease.
Why Is Stratus Being Monitored?
Even though it isn’t more dangerous right now, Stratus is spreading quickly—and that alone is enough reason to monitor it closely.
The WHO notes that XFG exhibits “marginal additional immune evasion” compared to previously circulating strains. In plain terms: it might slip past some parts of the immune system, but not enough to trigger alarm bells. Still, it has been seen in multiple countries, especially in South-East Asia, where some upticks in hospitalizations have occurred.
That doesn’t mean we’re on the brink of another global surge. It does mean that global health systems remain vigilant, especially as colder months approach in the Northern Hemisphere.
How to Protect Yourself from New COVID Variants?
No matter the strain—whether it’s Delta, Omicron, or now Stratus—the same precautions still apply:
- Stay up to date on vaccinations, including boosters recommended by your local health authority.
- Test when symptomatic, especially if you’re at high risk or around vulnerable individuals.
- Practice good hygiene: wash your hands, cover your cough, and sanitize high-touch surfaces.
- Wear a mask in crowded indoor spaces if transmission is high in your area.
- Take hoarseness seriously if it appears alongside other symptoms—especially if you’ve had recent exposure.
You might be asking: If Stratus isn’t more deadly, why should I care? Here’s why. Pandemics don’t end with a bang—they evolve quietly. Variants like Stratus remind us that SARS-CoV-2 is still adapting, and we need to stay informed even when things seem calm. By recognizing symptoms early, getting tested, and staying up to date on guidance, we help protect not only ourselves but the most vulnerable among us.
So no, Stratus isn’t a reason to panic but it is a reason to pay attention because COVID hasn’t disappeared—it’s just changed its voice.
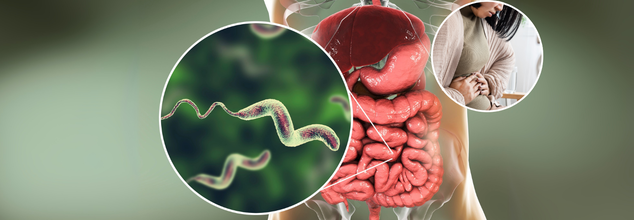
Diarrhoea Outbreak In Odisha Kills Two And Over 140 Hospitalised; Is It A Monsoon Menace Or Public Negligence?
Credits: Canva
A diarrhoea outbreak hit Ganjam district's Ustapalli village in Odisha, India, killing two individuals and hospitalizing more than 140. The outbreak was initially reported from Digapahandi block over the weekend. In a span of only three days, local hospitals were overwhelmed by patients with symptoms such as acute dehydration, severe stomach cramps, and vomiting.
There are ten patients in critical condition who have been shifted to MKCG Medical College and Hospital in Berhampur for intensive care. While medical personnel are deployed on the ground actively, the root cause is still being investigated. Preliminary suspicion is for contaminated drinking water, with test results on samples taken shortly.
“We’ve collected water samples from the village and are awaiting test results,” a medical officer told local reporters. “Meanwhile, awareness drives on hygiene and safe water consumption have been launched.”
This is not an isolated incident. Just weeks earlier, eight districts across Odisha, including Jajpur and Balasore, experienced widespread diarrhoea and cholera outbreaks. That previous wave of infections claimed at least 15 lives and affected over 1,500 individuals.
The Central team sent to Odisha validated the cause of the crisis: microbial contamination of water sources. Laboratory tests revealed several samples positive for E. coli and Vibrio cholerae — the cholera-causing bacterium. Significantly, 16 of 37 faecal samples from Jajpur were found positive for V. cholerae.
In recent cases, suspicion has also been cast on locally bottled drinking water consumed during public feasts in communities. Such small-scale bottlers frequently slip through food safety inspections, yet their products are widely distributed during public festivals.
How Can Diarrhoea Become Deadly?
On the surface, diarrhoea appears almost trivial — a symptom so easily treated with rest and fluids that it will go away on its own. But when it occurs in resource-poor areas or during outbreaks, this ubiquitous symptom can swiftly be deadly.
What is most dangerous about diarrhoea is how quickly it can dehydrate the body. The body loses water and critical electrolytes, resulting in such complications as low blood pressure, kidney failure, or death — more so for children, the elderly, and those with other ailments.
As per the CDC, diarrhoea-inducing pathogens — such as rotavirus, norovirus, and cholera — usually transmit through contaminated water or food. Rotavirus alone accounts for 40% of diarrhoea hospitalizations among children aged five years and under around the world.
In Odisha's situation, the lethal mix of unsafe drinking water, substandard sanitation infrastructure, and lack of access to healthcare continues to render diarrhoeal diseases a nagging public health issue.
Why Are Diarrhoea, Cholera Threats Resurfacing Frequently?
Cholera, previously much relegated to the fringes of public health issues in India's coast and cities, is now beginning to reappear. What started as a monsoon-led outbreak in tribal areas has now begun to hit semi-urban and urban coastal areas — a cause for concern.
Odisha's tribal areas have always been water-borne disease hotspots because of poor sanitation and lack of access to safe water. But this year's statistics indicate the issue is spreading beyond these confines.
Specialists consider this could be attributed to the effects of climate change, fast-paced urbanization without corresponding infrastructure, and declining oversight of food and water safety at local events. Complicating matters further is the development of antibiotic resistance in cholera strains — a cause for concern observed in previous outbreaks in the country.
Government Response: Quick Action But Enduring Gaps
Following these outbreaks, the Odisha government has gone into high gear. Rapid response teams, with paramedics and top health authorities, have been sent out to districts. Health Secretary Aswathy S has set aside no-nonsense orders, even suspending a doctor for medical misconduct in the midst of the crisis.
Central health authorities, in the meantime, have suggested immediate action: water purification at the point of distribution, raids on illicit bottlers, and hygiene campaigns.
But implementation lags exist. Residents in some of the impacted blocks complain of sporadic availability of clean water and an overburdened health system that cannot keep up with the demand. In a state celebrating one year of governance, the public health machinery is now under intense scrutiny.
Though this tale is set in an eastern Indian village, its consequences reverberate far beyond. Diarrheal illnesses claim almost 1.6 million lives annually across the world, ranking as one of the world's top 10 killers — The Lancet's 2019 Global Burden of Disease study reports.
The return of cholera and other water-borne diseases such as in Odisha is symptomatic of a larger pattern: how climate uncertainty, inadequate infrastructure, and poor regulatory environment can revive old vulnerabilities.
It also serves as a warning for travelers, especially those heading to developing regions. Though vaccines for rotavirus and cholera are available, they aren’t foolproof. Practicing safe food and water habits — drinking boiled or bottled water, avoiding raw foods, and washing hands regularly — remains essential.
Is It a Monsoon Menace or Public Negligence?
The monsoon rains are usually blamed for India's seasonal illnesses, but in the case of cyclical diarrhoea and cholera outbreaks such as the one that is currently taking place in Odisha's Ganjam district, it's not only nature that is to blame — human complacence has a much bigger role to play.
Yes, there is a possibility that heavy rain could clog sanitation systems, inundate water sources, and contribute to microbial contamination. But these situations are not unprecedented. What is worrying is the failure of the state to prepare when they are aware of such patterns. Outbreaks in eight districts within weeks — with water contamination, E. coli, and Vibrio cholerae detected — indicate systemic failures in water safety management and public health surveillance.
Another glaring lacuna is the consumption of cheap, unregulated packaged drinking water at public gatherings like festivities. Such local bottlers usually go undetected by quality checks, and food safety officials are unaware of them. Over and above that are substandard infrastructure, ineffective enforcement of hygienic standards, and little awareness campaign at the community level, and the result is nothing short of a disaster waiting to happen.
Labelling it merely a monsoon issue exculpates the guilty. This epidemic is a sign of systemic oversight, rather than seasonal bad luck. Clean water is a right, not a privilege — and until that's regarded as an absolute, these "monsoon" epidemics will keep killing.".
© 2024 Bennett, Coleman & Company Limited

