- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Is 'Stratus' COVID Variant The Pandemic’s Next Chapter In 2025 With New Symptoms?

Credits: Health and me
Nearly five years after COVID-19 first upended daily life, the virus continues to evolve. The latest variant gaining attention is called Stratus — a name already making its way through news headlines, social media chatter, and public health briefings. First flagged in multiple U.S. states and several countries in late 2024, Stratus is now showing steady growth in reported cases across the globe.
What makes it different? Scientists say Stratus belongs to the Omicron family, but carries a distinct set of mutations that may affect how it spreads and the symptoms it causes. While early data is still emerging, its trajectory has many experts urging caution without panic.
Stratus (officially XFG) popped up on researchers’ radar in early 2025 as a genetic recombination of two prior COVID-19 strains—LF.7 and LP.8.1.2. By late spring, it accounted for nearly 23% of global cases, and hovered at around 14% in the US. In England, its rapid climb—from 10% to 40% prevalence in mere weeks—warranted media nicknames like “Frankenstein variant” and set off alarm bells about just how fast it can spread. The World Health Organization currently lists Stratus as a variant under monitoring, citing its concerning immune-escape mutations but noting the overall public health risk remains low for now.
Also Read: Ozempic Users Found To Age Back By More Than 3 Years, Finds New Trial; Peer Review Pending
How Stratus Is Spreading?
Stratus’ dominance across regions stems from its transmissibility, not its severity. Four key mutations in the spike protein may help it evade immunity—whether from past infection or vaccination—but there’s no sign yet that it causes more severe disease.
Data from the U.S. Centers for Disease Control and Prevention (CDC) shows Stratus now accounts for a growing percentage of new COVID cases nationwide, particularly in urban centers with high travel activity. Similar trends are being reported in parts of Europe, Asia, and Australia.
Unlike earlier variants that surged sharply, Stratus appears to be building momentum more gradually — but steadily. This slower curve may allow it to spread under the radar for longer, especially in areas where testing and genomic sequencing have scaled back since the height of the pandemic.
What Are Stratus Symptoms You Should Know
If you’ve been keeping track, sore throats—and not just any sore throat—have become Stratus’ odd signature. People describe it as scratchy or raspy tones, easily mistaken for allergies or seasonal laryngitis. According to early clinical reports, the most common Stratus symptoms include:
- Persistent sore throat or hoarseness
- Headaches that last several days
- Nasal congestion and mild fever
- Fatigue that lingers beyond the acute phase
Some patients also report changes in smell or taste, but less frequently than with earlier variants. Shortness of breath and chest discomfort remain uncommon in vaccinated individuals but can occur in higher-risk groups.
Doctors stress that the full symptom spectrum may become clearer as more data is collected over the coming months.
COVID now spreads faster than before. According to updated medical data, incubation with Omicron-like variants—including Stratus—is often around 3 to 4 days—shorter than earlier strains’ 5–7 days. That means the usual advice is still relevant:
- If symptoms begin, test early—but not too early, since antigen kits may fail at onset.
- If negative, test again in 24–48 hours if symptoms persist.
- Most adults can end isolation after 5 to 7 days, provided symptoms are improving and any fever has resolved 24 hours without medication.
Expired or faulty tests? Double-check the control line on your test. If it doesn’t appear, the kit may be invalid—even if unused
Why New Variants Keep Appearing?
Viruses mutate — it’s part of their survival strategy. Each time SARS-CoV-2 infects someone, it makes copies of itself. Occasionally, those copies contain genetic changes that give the virus an advantage, such as spreading more efficiently or dodging parts of our immune response.
For Stratus, researchers are still analyzing whether its mutations make it more transmissible or better at evading immunity from vaccines or past infections. What’s clear is that population immunity, while strong, is not absolute — especially as antibody levels naturally wane over time.
Current COVID vaccines, including updated boosters targeting recent Omicron strains, are still expected to offer protection against severe illness from Stratus. However, breakthrough infections are possible, particularly in people who haven’t had a booster in the last 6–12 months.
Public health agencies continue to recommend boosters for older adults, people with weakened immune systems, and those working in high-exposure settings. Whether a Stratus-specific vaccine update will be needed remains to be seen.
Is Preventing Spread in 2025 A Reality?
We’re no longer in the emergency stage of the pandemic, but familiar prevention strategies still matter:
- Staying home if you’re sick
- Masking in crowded indoor spaces during surges
- Testing before visiting vulnerable individuals
- Keeping up to date on vaccinations
With reduced restrictions and increased global mobility, even moderate increases in transmission can ripple quickly through communities — especially during cold and flu season.
Researchers are tracking whether Stratus leads to more reinfections, if its symptoms last longer, and whether it’s linked to post-COVID complications like long COVID. Hospitals are monitoring for any shifts in admission patterns, particularly among children and older adults.
There’s also a focus on transparency: experts say real-time sharing of data across countries is essential for staying ahead of variant-driven waves.
Stratus isn’t dramatically different—no skyrocketed hospitalizations, no alarming new symptom profiles. But it does remind us that SARS-CoV-2 is still evolving, still engaging our resilience and still requiring vigilance.
You don’t need to panic. But staying informed, testing responsibly, masking when needed, and keeping vaccinations up to date that’s how we stay ahead of the next wave.
Epstein Files Raise Questions About Trump’s Memory Decline

Credit: Canva
Two years before Jeffrey Epstein killed himself in his New York jail cell, he claimed that US President Donald Trump may be suffering from dementia.
The United States Department of Justice recently released more of the harrowing Epstein Files that lift the lid on years' worth of horrific crimes committed by the convicted American child sex offender, serial rapist and human trafficker.
Among the files, eagle eyed readers found an email sent to journalist and Trump biographer Michael Wolff on Dec. 29, 2017, where Epstein claimed that Trump has begun showing signs of memory loss during his first presidency after he failed to recognize some of his own friends.
In the email to the Landslide author, the convict wrote: "Some at dinner with donald last night, were concerned about dementia. tons of makeup. did not recognize old friends.”
And Epstein isn't alone. Former White House chief strategist and Trump associate, Steve Bannon allegedly believed the president had "early-stage dementia," according to former 60 Minutes producer Ira Rosen’s book Ticking Clock.
Bannon claimed that Donald “had no attention span, didn't read, and now doesn't listen. He said Donald repeats himself a lot, telling the same story minutes after he told it before," per Rosen's account, which the former chief strategist later denied.
The producer also claimed in the book that “Bannon tried to build support to have Trump removed” from office due to his concerns.
Family Members Say Trump Has Dementia
Mary Trump, a well-known critic of her uncle who frequently speaks about him on her YouTube channel, has implied that he could be suffering from Alzheimer's disease, noting similarities to her late grandfather, who also suffered from the neurodegenerative disease.
As per UK Express, Mary highlighted that she has seen resemblances to Fred Trump, Donald's late father and former real estate magnate, who battled Alzheimer’s before passing away in 1999 at the age of 93.
Speaking last year, Mary recounted witnessing her grandfather’s decline and suggested that Donald sometimes doesn’t seem “oriented,” pointing to a particular look. Talking about her grandfather, she told New York Magazine: "One of the first times I noticed it was at some event where he was being honored. And I looked at him and saw this deer-in-the-headlights look, like he had no idea where he was."
In further remarks, Mary said she now notices what the publication described as “flashes” of her grandfather in her uncle when she sees him on stage, pointing out the same “deer-in-the-headlights” expression.
She added: "Sometimes it does not seem like he's aware of time or place. And on occasion, I do see that deer-in-the-headlights look."
Trump Rejects Memory Loss Claims
Despite multiple reports, Trump and his team have consistently rejected such claims, noting that he has “aced” three cognitive tests and there is no possibility of him having Alzheimer's disease.
In a conversation with the New York Magazine, Trump also reflected on his father’s diagnosis: "He had one problem. At a certain age, about 86, 87, he started getting what do they call it?"
His press secretary, Karoline Leavitt, supplied the term for Trump, who referred to it as an “Alzheimer’s thing,” asserting that he did not “have it.” The health of the 79-year-old has been the subject of much public speculation recently, with observers noting bruises on his hands, what appear to be swollen ankles, and rambling speech.
READ MORE: New FDA Approved Blood Test Can Predict Alzheimer’s Disease Before Symptoms Appear
What Is Alzheimer’s Disease?
Alzheimer's disease is one of the most common forms of dementia and mostly affects adults over the age of 65.
About 8.8 million Indians aged 60 and above are estimated to being living with Alzheimer's disease. Over seven million people in the US 65 and older live with the condition and over 100,00 die from it annually.
Alzheimer's disease is believed to be caused by the development of toxic amyloid and beta proteins in the brain, which can accumulate in the brain and damage cells responsible for memory.
Amyloid protein molecules stick together in brain cells, forming clumps called plaques. At the same time, tau proteins twist together in fiber-like strands called tangles. The plaques and tangles block the brain's neurons from sending electrical and chemical signals back and forth.
Over time, this disruption causes permanent damage in the brain that leads to Alzheimer's disease and dementia, causing patients to lose their ability to speak, care for themselves or even respond to the world around them.
While there is no clear cause of Alzheimer's disease, experts believe it can develop due to genetic mutations and lifestyle choices, such as physical inactivity, unhealthy diet and social isolation.
Early symptoms of Alzheimer's disease include forgetting recent events or conversations. Over time, Alzheimer's disease leads to serious memory loss and affects a person's ability to do everyday tasks.
There is no cure to this progressive brain disorder and in advanced stages, loss of brain function can cause dehydration, poor nutrition or infection. These complications can result in death.
Measles In DC: Health Officials Warn of Possible Exposure After National March for Life Events
Credits: Canva
Health officials in Washington, D.C. are warning that confirmed cases of measles may have spread during this year’s National March for Life rally and related events held in the capital late January. The annual anti-abortion gathering drew thousands of people to the National Mall and surrounding areas, raising concerns about potential large-scale exposure.
The D.C. Department of Health said it is actively working to identify individuals who may be at risk after learning that several people who later tested positive for measles were present in the city while contagious.
“DC Health was notified of multiple confirmed cases of measles whose carriers visited multiple locations in the District while contagious,” the agency said in a statement on Sunday. Officials are now contacting people who were at those locations during the exposure window.
Measles In DC: Transit Hubs and Campuses Among Exposure Sites
According to DC Health, potential exposure sites span a wide range of busy public locations between January 21 and February 2. These include Ronald Reagan Washington National Airport, Union Station, an Amtrak Northeast Regional train, and multiple stops within the city’s Metro subway system.
Health officials also flagged visits to the Basilica of the National Shrine of the Immaculate Conception and Catholic University as part of the exposure timeline. Given the volume of visitors moving through these spaces daily, authorities say the risk of wider spread cannot be ruled out.
Measles In DC: Hospital Issues Public Health Notice
Children’s National Hospital has also issued a public health notice after a confirmed measles patient from Virginia visited its Emergency Department on February 2 while infectious. The hospital said it is coordinating with public health authorities to identify and notify anyone who may have been exposed during that time.
Measles is highly contagious and can remain airborne for up to two hours after an infected person leaves an area, making hospital settings particularly vulnerable.
Measles In DC: Surge Across the United States
The situation in Washington comes as the United States faces its largest measles outbreak in decades. According to the Centers for Disease Control and Prevention, 733 confirmed cases have been reported across 20 states so far this year. The CDC says about 95 percent of those cases involve people who were unvaccinated or whose vaccination status is unknown.
South Carolina remains one of the hardest-hit states. Its outbreak began in October 2025 and has now surpassed earlier outbreaks elsewhere in the country. State health officials reported 44 new cases on Friday, bringing the total to 920. While the pace of new cases has slowed slightly, officials continue to warn of possible exposure at public places such as a Target store in Taylors and a Social Security Administration office in Spartanburg, where the outbreak is centered.
Measles In DC: Vaccination Urged Amid Global Concern
Speaking on CNN’s State of the Union, Centers for Medicare and Medicaid Services Administrator Mehmet Oz urged Americans to get vaccinated against measles. While recent federal policy changes have rolled back recommendations for some vaccines, guidance on measles immunization remains unchanged.
International health authorities are also watching closely. The World Health Organization’s Pan American Health Organization has invited U.S. officials to a meeting in April to review the country’s measles elimination status, which is now under threat.
D.C. health officials are urging anyone who may have been exposed and is not fully vaccinated, pregnant, or immunocompromised to contact a healthcare provider immediately.
Epstein Files: A Chat With Urologist Shows Stendra Was Prescribed To Jeffery Epstein; Why Did This Name Come Up?

Credits: DOJ, Canva, AI-generated and modified
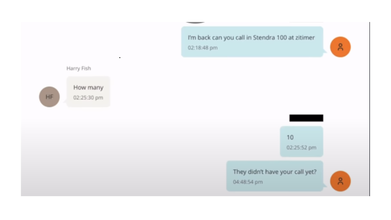
The latest release by Department of Justice (DOJ) on Epstein Files show a chat between the late sex offender and financer Jeffery Epstein and Harry Fisch, a urologist. The message from Epstein reads: "I am back can you call in Stendra 100 at zitimer".
Epstein Files: What Is Stendra Used For?
Stendra, which is a common brand name for avanafil, is a commonly used medicine for erectile dysfunction. This is a condition where a man has trouble getting or keeping an erection. It can also be used for other conditions as determined by a healthcare provider.
It works by blocking an enzyme in the body called PDE5, which helps relax certain blood vessels. This also increases blood flow to the penis when aroused, and makes it easier to get and keep an erection.
Epstein Files: Did Jeffery Epstein Have An STI?
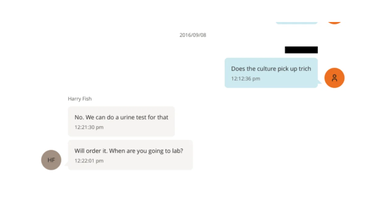
Another chat thread between the two reveal that Epstein was worried if he had caught 'trich' or trichomoniasis. The chat from Epstein reads: "Does the culture pick up trich", to this Harry replies: "No. We can do a urine test for that".
Trichomoniasis or trich is a common, curable sexually transmitted infection in men caused by the parasite Trichomonas vaginalis. While many men are asymptomatic, they can still transmit the infection.
Common symptoms of trich are:
- Burning after urination
- Burning after ejaculation
- Itching
- Penile discharge
In another screenshot of the chat, Harry responded that he had ordered a Trich urine test and the test was negative on 9/6/16.
However, based of the DOJ documents and report by The Times, a blood test in 2016 reported Epstein had tested positive for gonococcus (GC), or gonorrhea.
Epstein Files: What More Do These Documents Reveal About His Health?
Epstein had 'very low' testosterone levels, and appeared to have cryogenically frozen his sperm, reported The Times, based on the medical records released by DOJ.
A urology test also showed that his testosterone levels were well-below normal levels in 2016. On this, Epstein noted that it had been the "same for ten years".
His reported levels ranged between 65 and 150 nanograms per deciliter (ng/dL), far below the normal range of about 350 to 1,000 ng/dL, and warrant prompt medical consultation to identify the underlying cause, according to the Cleveland Clinic.
In a 3am email dated April 24, 2015, Epstein, who was 62 at the time, wrote to one of his doctors, Dr Bruce Moskowitz: “As you can see from the time stamp my sleep pattern is not wonderful. I am hesitant to start a regimen of hormones. my low testosterone has been there for 15 years. mechanic view is that it has caught up to me?”
Among the several doctors he consulted, one advised Epstein to use testosterone replacement therapy along with Clomid, a drug that blocks estrogen receptors in the brain and stimulates the body to produce more testosterone. In a 2016 email to Dr Peter Attia, Epstein said he had stopped taking Clomid, calling it a “giant mistake.” “Stopped the clomid the water retention and fat around the waist made it as if i was pregnant,” he wrote.
© 2024 Bennett, Coleman & Company Limited

