- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
'Let Bird Flu Spread,' Advises RFK Jr. To Build Herd Immunity

Credits: Getty Images and Canva
Robert F Kennedy Jr, who is the United States Secretary of Health and Human Services HHS, has remained on top of the news headlines with his never-ending controversial comments. In another controversial proposal, he has asked to let bird flu naturally spread through poultry farms. This has raised alarms among scientists who say this move could be inhumane and dangerous.
He suggested that instead of culling the birds, the farmers should allow the virus to run its course through the flock to identify naturally immune birds. He told Fox News, "We can identify the birds and preserve the birds that are immune to it."
While Kennedy has no direct control over the farms, the Agriculture Secretary Brooke Rollins has also taken an interest in testing this idea. He told the CBS, "There are some farmers that are out there that are willing to really try this on a pilot as we build the safe perimeter around them to see if there is a way forward with immunity."
However, experts, including the veterinary experts say that this could in fact backfire. Dr Gail Hansen, who was a former state veterinarian for Kansas told The New York Times, "that's a really terrible idea for any one of a number of reasons."
Bird Flu Impact In US
Bird flu in the US has affected more than 166 million birds since 2022. At such a rate, experts warn that allowing the virus to run through the flock would increase the risk of it mutating. However, the administration thinks otherwise, "Culling puts people at the highest risk of exposure, which is why Secretary Kennedy and N.I.H. want to limit culling activities. Culling is not the solution. Strong biosecurity is,” said Emily Hilliard to The Times, who is the deputy press secretary at the Department of Health and Human Services.
Experts across however have suggested against it. They have even predicted that if this is allowed, it would kill 100% of all turkeys and chicken. The reason is because the way birds are raised now do not have a lot of genetic variability. They are all basically the same bird. However, what Kennedy had suggested was for poultry to have a natural immunity, almost like the concept of "herd immunity". However, it cannot be the case when birds do not have a diverse genetics.
New Developments In Bird Flu
To give a ray of hope, scientists have now created a handheld sensor that can quickly detect H5N1 in the air, which could potentially stop the outbreak before they spread.
The findings of this handheld sensor is published in journal ACS Sensors, which noted it to be a low-cost, highly sensitive and workable sensor.
This research was led by Rajan Chakrabarty, who is the leader of the Aerosol Interdisciplinary Research(AIR) group at Washington University. His team developed the sensor using electrochemical capacitive biosensor (ECB) technology. The ECB features a thin network of nanocrystals and graphene oxide, with special probes that attach to bird flu particles.
This is a built-in-air sampler, which collects airborne virus droplets and turns them into a liquid sample. When the virus binds to the sensor, it changes the device's ability to hold electrical charge. This allows the scientists to measure virus levels.
In lab tests, the ECB sensor detected the H5N1 virus within five minutes. It was also sensitive enough to identify 95 viral copies per 35 cubic feet of air. This is a level that researchers say should be "sensitive enough to detect the presence of H5N1 below the virus' infectious dose."
Read more on Bird Flu, here.
Jharkhand Education Minister Ramdas Soren Suffers Brain Hemorrhage, Airlifted To Delhi
Credits: Canva
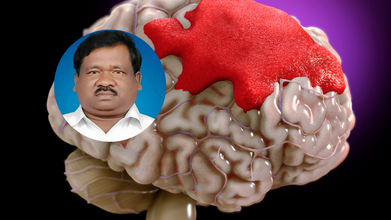
Jharkhand Education Minister Ramdas Soren remains in critical condition after suffering a severe brain injury following a fall in the bathroom at his residence early Saturday morning.
According to officials, the minister was initially admitted to a hospital in Jamshedpur, where doctors detected a blood clot in his brain. He was later airlifted to a private hospital in Delhi for more advanced treatment.
Health Minister Irfan Ansari confirmed the incident and said that Soren’s condition deteriorated rapidly after the fall. “Ramdas Soren’s health suddenly declined. He suffered a serious brain injury and internal bleeding in the brain. I have been in constant touch with his family and am monitoring the situation closely,” Ansari said.
Airlifted to Delhi for Advanced Care
After initial treatment in Jamshedpur, the decision was made to transfer the minister to Delhi for more specialized care. Former Union Minister and senior BJP leader Arjun Munda, who was present at Sonari Airport during the airlift, said, “I have spoken to the director of Delhi Apollo Hospital, and he has assured that treatment will begin as soon as the minister reaches. The doctors have diagnosed a brain hemorrhage due to a sudden increase in pressure. His condition is critical, but we are hopeful.”
On Saturday evening, the Delhi hospital issued a statement confirming that the minister was on life support and under the care of a multidisciplinary team of senior specialists. “A close watch is being kept on all vital parameters,” a spokesperson for the hospital said.
Understanding Brain Hemorrhage: What Happened to Ramdas Soren?
A brain hemorrhage, commonly known as a brain bleed, occurs when there is bleeding either inside the brain tissue or in the space between the brain and the skull. It is a medical emergency that can cause damage by increasing pressure on the brain, reducing oxygen supply, and killing brain cells.
Doctors categorize brain hemorrhages based on where the bleeding occurs:
Bleeding Outside Brain Tissue (Within Skull)
Epidural Hemorrhage: Occurs between the skull and the outer membrane (dura mater)
Subdural Hemorrhage: Bleeding between the dura mater and the arachnoid membrane
Subarachnoid Hemorrhage: Bleeding between the arachnoid and the innermost membrane (pia mater)
Bleeding Inside Brain Tissue
Intracerebral Hemorrhage: Bleeding within the brain’s tissues
Intraventricular Hemorrhage: Bleeding into the brain’s internal cavities that hold cerebrospinal fluid
In Soren’s case, doctors suspect a form of intracerebral or subdural hemorrhage, given the mention of clotting and pressure build-up.
Symptoms and Risks
Brain bleeds can come on suddenly and include symptoms like severe headache, nausea, vomiting, confusion, weakness or numbness (especially on one side of the body), vision problems, dizziness, seizures, slurred speech, and even coma. A particular warning sign is the so-called “thunderclap headache”, a sudden, intense pain that can signal a subarachnoid hemorrhage.
When not treated immediately, such bleeds can be fatal or lead to long-term neurological complications. In some cases, surgery is required to relieve pressure and remove clots.
What’s Next?
As of Saturday night, Ramdas Soren continues to remain on life support. No additional medical bulletins have been released since his transfer to Delhi, but officials have indicated that his condition is still being evaluated. “We are hoping for positive progress, but his condition remains critical,” said Arjun Munda.
Family members and close colleagues are at the hospital, and the Jharkhand government has said it will provide all necessary support for his treatment.
The political and public community has expressed concern and extended wishes for the minister’s speedy recovery. Further updates are awaited from the hospital and health authorities.
FDA Escalates Recall Of 64,800 Lbs. of Butter Over Undeclared Allergen

Credits: Canva
In a growing food safety alert, the U.S. Food and Drug Administration (FDA) has escalated a butter recall to a Class II risk level following concerns over undeclared allergens. The product in question, European Style Butter Blend manufactured by Bunge North America Inc., was found to contain milk that was not listed on the packaging label.
Class II Recall Indicates Moderate Health Risk
The risk reclassification, issued on Wednesday, July 30, places the product under the FDA’s second-highest warning level. According to the FDA, a Class II recall involves “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
While no illnesses or allergic reactions have been reported so far, the undeclared presence of milk poses a potential health hazard to people with dairy allergies or lactose intolerance.
Initial Voluntary Recall Announced in Mid-July
The recall began as a voluntary measure by Bunge on July 14, when the company announced it was pulling approximately 64,800 pounds, or 1,800 cases, of its one-pound butter blocks from shelves. The recalled butter was packed in white paperboard cases, each containing 36 one-pound blocks.
The affected products carry the lot code 5064036503 and were shipped to 12 distribution centers across the United States and one in the Dominican Republic.
Why the Undeclared Milk Is a Serious Concern
Milk is one of the nine major food allergens identified by the FDA, alongside eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, soybeans, and sesame. The FDA mandates clear labeling of such allergens because exposure, even in small amounts, can cause a range of reactions, from mild discomfort to life-threatening symptoms.
Food-related allergic reactions may include hives, facial swelling, vomiting, coughing, and skin irritation. More severe responses can result in anaphylaxis, a rapid-onset, whole-body allergic reaction that may lead to shock and, in extreme cases, death.
According to the Mayo Clinic, anaphylaxis occurs when the immune system floods the body with chemicals in response to an allergen. This can cause a sudden drop in blood pressure, narrowing of the airways, and potential organ failure if not treated immediately.
FDA Reiterates Importance of Allergen Labeling
In light of the recall, the FDA has emphasized the importance of allergen labeling and said it continues to enforce regulations requiring companies to clearly list all ingredients and potential allergens on packaging.
“More specific labeling requirements exist for foods that can cause allergies or other hypersensitivity reactions,” the agency stated. “These rules are designed to prevent accidental consumption of allergens and to protect consumers with dietary restrictions.”
The FDA also advised that anyone who experiences symptoms of an allergic reaction after consuming the recalled butter should “stop eating the food immediately, evaluate the need to use emergency medication (such as epinephrine), and seek medical attention.”
Company Yet to Comment
As of August 2, Bunge North America has not issued an updated public statement in response to the FDA’s reclassification and did not respond to a request for comment.
Food Safety Under Scrutiny Amid Other Recent Recalls
This butter recall follows a string of other high-profile food safety incidents this year. In recent weeks, more than 110,000 cases of popular chocolate ice cream bars were recalled across 23 states. Target-branded baby food was also pulled from shelves for containing “elevated levels of lead.”
Could This New Alzheimer’s Drug Buy Patients Four More Good Years? Here Is What We Know

Not forever, but what if you could press pause on Alzheimer’s just long enough to enjoy a few more good years? That is the tantalising promise behind a new drug called lecanemab, hailed as a game-changer in the fight against dementia.
The drug has already been licensed for use in the UK after trials showed it could slow the pace of decline in people with early-stage Alzheimer’s. But new long-term findings are turning cautious hope into something stronger: patients who stayed on lecanemab for four years experienced a noticeable delay in the disease's progression. Some even showed no decline at all.
How It Works
Alzheimer’s is known for its slow but relentless grip on memory and cognition, typically marked by the build-up of sticky proteins in the brain. Lecanemab targets tau, a protein that increases as the disease worsens.In the initial 18-month trial, the drug delayed Alzheimer’s progression by just under six months. That might not sound like much, but it’s the long game that matters here. Among 478 patients who remained on the drug for four years, the average delay before their disease advanced to the next stage stretched to almost 11 months.
Even more striking: 69 per cent of those with low levels of tau saw no decline at all over the four years. And over half in that same group actually improved their cognitive scores.
A Slow Slide Instead of a Steep Drop
Typically, people with mild Alzheimer’s see their scores on memory and function tests worsen by one or two points each year. But for those taking lecanemab, the total decline across four years was just 1.75 points. That’s a major shift in the rhythm of the disease, changing it from a downhill tumble to a slow shuffle.
Professor Christopher Van Dyck, who led the study at Yale School of Medicine, puts it simply: “You will get worse over time, but it will take longer to get there.” That extra time could mean more independence, more connection with loved ones, and more living.
Why Early Treatment is Key
The benefits weren’t evenly distributed. Patients who had less evidence of Alzheimer’s pathology, that is, fewer early changes in the brain, showed the most striking outcomes. In other words, the earlier you start treatment, the better your odds of preserving function.This makes a strong case for early diagnosis and intervention, which could shift the way we approach Alzheimer’s care. No longer is it just about managing symptoms; it’s about changing the trajectory of the disease.
Not a Cure, But a Clear Step Forward
Lecanemab isn’t a miracle cure. It doesn’t reverse Alzheimer’s, and it’s not suitable for all patients. But experts say it’s a major milestone. Reportedly, this is the first wave of disease-modifying treatments and there’s still plenty to understand.
Other Contenders in the Ring
Lecanemab isn’t the only drug showing promise. A similar treatment called donanemab was tested over a three-year period, though it was only administered for 18 months. Still, the results were encouraging: patients on the drug gained an extra six to 12 months before their disease progressed.
That might not sound earth-shattering, but in a condition where time is everything, even a few more months of clarity and connection can be priceless.
The research is still evolving, but the signs are encouraging. With continued trials, this could be the start of a new chapter in dementia treatment, one where patients and families have more time to prepare, more time to enjoy life, and more hope than ever before.
© 2024 Bennett, Coleman & Company Limited

