- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
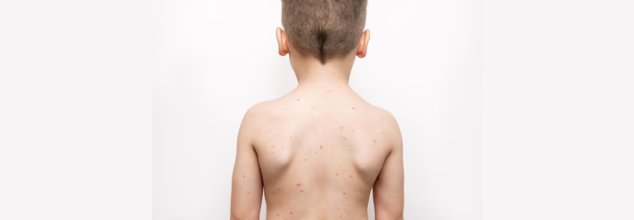
Measles Cases Hit Record Levels—Highest Surge Since US Declared Disease Eliminated; Is This America's Public Health Failure?
Credits: Freepik
For the first time in a quarter-century, the United States is seeing more cases of measles during a single year than at any point since the disease was last declared eliminated in 2000. A startling 1,277 cases were reported as of mid‑2025—already more than the former yearly record of 1,274, which was set in 2019. Adding to alarms, three lives—two kids in Texas and an adult in New Mexico—have already been lost this year, equaling the number of U.S. measles deaths since elimination.
This comeback highlights both the vulnerability of public health gains and the lethal effects of waning vaccination levels.
What "Elimination" Meant—and Why It's Slipping Away?
In 2000, when the U.S. marked measles elimination, it meant zero sustained domestic transmission for over a year—a feat achieved due to successful vaccination and disease surveillance. A strong two-dose schedule of the measles-mumps-rubella (MMR) vaccine, accessible since the 1970s, fueled that achievement.
But since 2021, national school-age MMR coverage has slipped below the all-important 95% herd immunity bar that experts say is critical to widespread protection. More than 125,000 kindergartners alone had exemptions from one or more mandatory vaccines during the 2023–24 school year. That set the stage for contagion to spread—particularly in clusters of unvaccinated communities.
In West Texas, an undervaccinated area in late January sparked a huge outbreak. Over 750 cases have been traced to the region, spreading to Oklahoma and New Mexico and prompting an early vaccination campaign. Counties reacted by administering early doses of MMR as young as six months old, and in Texas, early infant vaccination jumped—the rate was eight times higher than in 2019, according to Truveta health data.
Communities rallied with pop-up vaccination sites, state-level policy interventions, and an increased uptake of MMR. Nevertheless, smaller outbreaks in 38 states and 27 discrete clusters indicate a more pervasive susceptibility.
Why Cases Are Skyrocketing Now?
Waning MMR Coverage: Sustained vaccine skepticism and exemptions have eroded herd immunity in communities.
Highly Infectious Virus: Measles spreads more quickly and widely than many respiratory diseases—up to 90% infection of susceptible close contacts. It only takes one contagious person to initiate large outbreaks.
Global Spread: Measles is endemic throughout the world. Imported cases in countries where the virus is circulating continuously are the lifeblood of ongoing outbreaks.
Local Susceptibility: With an unvaccinated cluster present, the virus circulates nearly unimpeded.
In 2019, misinformation-driven outbreaks infected Orthodox Jewish communities in New York. Now, the epicenter is Texas, but the virus is spreading extensively—eliciting extensive alarm.
While the epicenter is still West Texas, the virus has now spread in several states:
New Mexico & Oklahoma: Connected to initial outbreak, with overlapping transmission chains.
Kansas: 83 cases, all but one related to primary Texas outbreak.
Wyoming: Expressed a measles case for the first time since 2010—an unvaccinated kid in rural Casper.
Utah, Michigan, Florida: New instances of travel-associated cases have surfaced, emphasizing the national risk.
Public health authorities are cautioning that if transmission persists after January 2026, the U.S. will forfeit its measles elimination status, which is reversing a big public health achievement.
Why Is Measles A Continental Health Threat?
The Reuters Health update is a reflection of the U.S. crisis. Pan American Health Organization (PAHO) indicates a 29-fold increase in measles in the Americas compared to last year. There have been 3,170 cases in Canada since 1998 and Mexico had 2,597 cases in 2025—with 10 related deaths. Mennonites and other isolated communities' vaccine skepticism is driving the comeback.
The CDC has estimated a mere 8% of the 2025 cases in fully or partially vaccinated persons. In other words, more than 90% infected were unvaccinated. Hospitalizations have been glaringly high: 155 patients, including numerous young children. Specifically, 28% of these cases are in children under age 5—the exact age group that the MMR vaccine is supposed to protect.
MMR vaccine is one of the most effective medical tools ever—93% effective after one dose, and as much as 97% after two. Yet its effectiveness hinges on widespread coverage, not simple use. There are several forces at play:
Vaccine Hesitancy & Misinformation: Sustained disinformation efforts—across social media and conspiracy networks—chips away at confidence in vaccines.
Policy Vacuum: The U.S. lacks a permanent CDC director. HHS Secretary Robert F. Kennedy Jr. surprised with a pro-vaccine public statement this spring, but his past record of vaccine skepticism and disregarding CDC advisory panels erodes faith.
Educational Shifts: Additional states have broadened vaccine exemption policies, granting greater refusal under medical, religious, or philosophical reasons.
At levels of community coverage below 95%, outbreaks become nearly inevitable.
Has U.S. Failed its Children?
Others suggest 2025 represents a systemic public health collapse. The pathogen's general return is a reflection not of biology but policy abandonment and frayed solidarity. Vaccination is not just individual defense—it's a social compact.
Others notice a silver lining: almost universal alarm, rapid policy response, and vaccination catch-up campaigns are proving the system responsive—if communities reassert collective responsibility.
This isn't about erecting barriers, but about renewing the networks of trust that allow vaccines to protect whole communities.
We stand at a crossroads, The resurgence of measles illustrates how progress can vanish when vigilance does. Yet while 2025 has set new records, it also sparked renewed urgency—and rare moments of collective action.
We can't risk losing what was once seen as core public health. At the center of this battle is a simple, evidence-based fact: Vaccination saves lives—not only individuals, but communities. America has to choose: is this the time we repair the fissures in public trust and systems—or do we allow measles take back territory?
Trump Denies Health Concerns, Says Hand Bruise Was Caused By Aspirin

Credits: AP
President Donald Trump said he takes a higher dose of aspirin than his doctors recommend, blaming it for the bruises on his hands that have again drawn attention to his health. The remarks came during an interview with The Wall Street Journal published on Thursday.
“They tell you aspirin is good for thinning the blood, and I don’t want thick blood running through my heart,” Trump, 79, said. “I want thin blood flowing through my heart. Does that make sense?”
“I Want Thin Blood,” Trump Explains Why He Ignores Doctors’ Advice
Trump said his doctors prefer that he take a smaller dose, but he has chosen otherwise. “They’d rather I take the smaller one. I take the larger one. I’ve done it for years,” he said, adding that bruising is a known side effect, as per CNN
Bruising, Makeup, and Bandages Renew Questions About Trump’s Health
The interview marked one of Trump’s most detailed discussions with journalists about his health in recent years. Scrutiny has grown as questions persist about his age, stamina, and how transparent the White House has been about his medical condition.
Bruising on Trump’s right hand has been visible for months. CNN previously reported that it existed before his return to the White House. The issue drew more attention after Trump appeared to conceal the marks with makeup or bandages and often shielded his hand from cameras. Observers have also pointed to swelling in his legs and moments where he appeared to doze during public events.
Trump’s Doctor Confirms President Takes 325 mg of Aspirin Daily
Trump’s physician, Dr. Sean Barbabella, told the Journal that the president takes 325 milligrams of aspirin each day. According to the Mayo Clinic, low-dose aspirin therapy typically ranges from 75 to 100 milligrams, with 81 milligrams being the most commonly advised dose. The clinic notes that aspirin therapy can fall anywhere between 75 and 325 milligrams daily.
What Is Aspirin Used For?
Aspirin is widely used to thin the blood and reduce clot formation, lowering the risk of heart attack and stroke. However, it also increases the risk of bleeding. In recent years, medical guidelines have moved away from recommending daily aspirin for many adults, as the potential harms often outweigh the benefits. Some experts advise discontinuing aspirin entirely in people in their 70s.
Dr. Jonathan Reiner, a professor at George Washington University’s School of Medicine and Health Sciences and former cardiologist to Vice President Dick Cheney, said the explanation offered by Trump and his team raises further questions.
“It’s uncommon to see that kind of bruising with one aspirin a day,” Reiner said. “The question becomes whether the president is taking medications that have not been disclosed by the White House.”
White House Says Trump Is in “Exceptional Health”
Barbabella said on Thursday that Trump remains in excellent condition. In a statement to CNN, he said the president’s medical evaluations and lab results show strong metabolic health and that his cardiovascular condition is comparable to someone 14 years younger. “Overall, the President remains in exceptional health and fully capable of carrying out his duties as Commander in Chief,” Barbabella said.
Bruising Also Seen on Trump’s Left Hand During Recent Events
At several public appearances last week, Trump appeared with light discoloration or bruising on the back of his left hand as well, in addition to the more persistent bruising on his right hand. As per CNN, the White House has previously attributed the right-hand bruising to frequent handshaking combined with aspirin use, which can make bruises more likely to appear.
Why Daily High-Dose Aspirin Is Rarely Recommended
Reiner said that while 325 milligrams is not considered an extreme dose, there is no clear medical reason to take that amount every day. He explained that people with acute injuries, such as a sprained ankle, may be prescribed 325 milligrams every four hours, which would be considered a high dose.
“Aspirin has been studied at many doses,” Reiner said, as per NBC News. “Eighty-one milligrams offers the best balance between reducing clot risk and limiting bleeding. A higher dose increases bleeding risk without improving effectiveness. That’s why we don’t use it.”
He added that the broader concern is a lack of transparency. “All of this highlights how opaque the White House has been about the president’s health.”
Trump Clarifies October Medical Scan Was a CT, Not an MRI
During the interview, as per CNN, Trump also discussed a medical scan he underwent in October. At the time, he told reporters it was an MRI but declined to provide details, directing questions to his doctors.
Speaking to the Journal, Trump clarified that the scan was a CT. “It wasn’t an MRI,” he said. “It was less than that. It was a scan.” Barbabella said the CT scan was done “to definitively rule out any cardiovascular issues.” Last month, he released a memo stating that imaging of Trump’s cardiovascular and abdominal systems showed “perfectly normal” results.
In 2018, however, Trump underwent a coronary CT scan that revealed plaque buildup in his arteries, indicating moderate heart disease.
White House press secretary Karoline Leavitt defended the administration’s disclosures, saying the president’s doctors have always confirmed he received advanced imaging. She added that Trump himself has shared additional details and described him as “the most transparent and open president in history,” while criticizing former President Joe Biden.
Trump Addresses Leg Swelling Linked to Chronic Venous Insufficiency
Trump also spoke about swelling in his lower legs, which the White House announced in July was caused by chronic venous insufficiency, a common condition among older adults. Trump said he tried compression socks but stopped using them. “I didn’t like them,” he told the Journal.
He also suggested he has little interest in regular exercise. “I just don’t like it. It’s boring,” Trump said. “Walking or running on a treadmill for hours, that’s not for me.” The Journal questioned Trump about moments where he appeared to fall asleep during public appearances. During a Cabinet meeting last month, Trump closed his eyes for several seconds at a time. A similar moment occurred during a November 6 event in the Oval Office.
Trump denied falling asleep. “I just close my eyes. It’s very relaxing to me,” he said. “Sometimes they take a picture when I’m blinking, and that’s what people see.”
President Downplays Hearing Concerns
Asked about his hearing, Trump said he only struggles when many people are talking at once and dismissed broader concerns. Before and after the election, Trump repeatedly questioned Biden’s fitness to serve, even suggesting Biden was unaware of documents signed in his name using an autopen. Biden has denied the claim.
Biden later exited the 2024 presidential race following a widely criticized debate performance that intensified concerns about his health and ability to remain in office.
India Bans Nimesulide Drugs Over 100mg Over Health Concerns, All That You Need To Know
Credits: iStock
Nimesulide (over 100 mg), a commonly used non-steroidal anti-inflammatory drug (NSAID) which is prescribed for pain and inflammation is now banned in India. This is to safeguard public health.
What Exactly Happened? The Centre has prohibited the manufacture, sale and distribution of oral formulations of nimesulide which contains more than 100mg, with immediate effect, across the country.
As per the government notification, oral formulations of nimesulide that contains more than 100 mg have been banned as such high-dose could be risky for human health. The order states that safer alternatives to this drug is available in market, and thus in public interest, the continued availability of this drug at such high dosage is not justified.
Also Read: Superbug Fungus Candida Auris Is Spreading In The U.S., Check Where Cases Are Rising Fastest?
The ban is issued under the Section 26A of the Drugs and Cosmetics Act, 1940. This section empowers the Centre to restrict or prohibit drugs that pose a risk to patients. The government also consulted the Drugs Technical Advisory Board (DTAB), which is also country's apex advisory body on technical matters related to drugs and cosmetics.
Why Did Government Ban Nimesulide?
The drug had long been under regulatory and medical scrutiny. The major concern is the liver toxicity, which is one of the side effects of this high-dose drug. In fact the World Health Organization (WHO) has no included nimesulide in its Model List of Essential Medicines, all due to the global caution around it. Several other countries too have either restricted or completely withdrew the drug.
The Ministry of Health and Family Welfare in a notification said that the Central government is "satisfied that the use of all oral formulations containing Nimesulide above 100 mg in immediate release dosage form is likely to involve risk to human beings and that safer alternatives to the said drug are available".
Also Read: Indore Water Contamination Linked to E. coli and Klebsiella Bacteria — What Are They?
The restriction on certain nimesulide formulations follows recommendations made by the Indian Council of Medical Research (ICMR) after reviewing the drug’s effects in adults. These recommendations were accepted by an expert committee under the apex drug regulator. Nimesulide is known to cause liver toxicity in some cases.
ICMR had also advised that nimesulide be used only as a second-line treatment when other medicines fail or cannot be prescribed. It recommended against its use in pregnant or lactating women, those planning pregnancy, and patients with liver or kidney impairment. The drug should also not be used alongside other medicines that are toxic to the liver or kidneys. Nimesulide is already banned for children under 12 years of age.
The committee has further asked ICMR to review the impact of the drug across different age groups, including adolescents and older adults.
Read: Cough Syrup Row: Death Toll Rise To 22 As 2 More Children Succumb
What Are The New Development On Drug Ban In India?
The Ministry also removed cough syrups from the list of over-the-counter medicines. While lozenges, pills or tablets for cough continue to remain on the list. This has come in the backdrop of 22 children who died in Madhya Pradesh after they consumed contaminated cough syrups.
Disclaimer: Health and Me's report is based on the notice issued in public knowledge and is not a substitute for prescribed drugs. Always consult your doctor before making changes in your medicines.
Explained: Indore Water Contamination Linked to E. coli and Klebsiella Bacteria — What Are They?

Credits: PTI and CDC
Indore water contamination is linked to fecal bacteria like E coli and Klebsiella, confirmed officials. These bacteria are known to cause severe vomiting and diarrhea. The death toll too have risen to 10, confirmed the local officials. Indore councillor Kamal Waghela said that two more persons died on Thursday night. The laboratory tests of the water supplied through the Narmada pipeline in Bhagirathpura, which is the epicentre of the outbreak confirmed E coli and Klebsiella bacteria to be the culprits.
Also Read: Australian Cricketer Damien Martyn in Hospital With Meningitis
What Happened In Indore That Led To Water Contamination?
The outbreak occurred due to lapses in civic infrastructure. Investigation revealed that a toilet constructed directly above a main drinking pipeline near a police outpost, without a mandatory safety tank resulted in the sewage mixing with drinking water.
Indore Municipal Corporation Commissioner Dilip Kumar said, “We have found that in case of the construction of the toilet, no safety tank was constructed beneath it. We are also probing the other lapses.”
What Is E. Coli - The Bacteria Found In Indore Contaminated Water?
Escherichia coli, or E.coli is a bacterium that lives in the human intestine and is one of the most common causes of foodborne illness.
The Centers for Disease Control and Prevention (CDC), these bacteria are commonly found in environment, food, water, and the intestines of people and animals. While most E.coli are harmless, some can make people sick with diarrhea, urinary tract infection, pneumonia, sepsis, and other illnesses.
E. Coli from Contaminated Water
WebMD writes that one can swallow E.coli from contaminated water, as in the case of Indore, a toilet was constructed directly above the main drinking water pipeline, without a mandatory safety tank, which may have led to the contamination. Studies have also shown that some E.coli could regrow even after chlorine treatment.
What Is Klebsiella - The Bacteria Found In Indore Contaminated Water?
CDC notes that Klebsiella is a type of gram-negative bacteria which is also found in human stool and can cause healthcare-associated infections or HAIs. They are also highly resistant to antibiotics and can also resist carbapenems, which is the last line of defense against multi-drug resistant bacterial infections.
Klebsiella can cause pneumonia, bloodstream infections, wound or surgical site infections and meningitis.
It can spread from person-to-person contact, which is one of the most common way of spreading. Other ways could also be from contact with contaminated water or soil, contact with contaminated equipment, for example, ventilators, breathing machines, or intravenous catheters. Klebsiella cannot spread through the air.
Also Read: Sewage Mixing With Drinking Water Kills 7 in Madhya Pradesh’s Indore, Over 100 Remain Hospitalized
What Actions Have Been Taken So Far In Indore?
As of now, the water supply Assistant Engineer (AE) has been suspended, and the sub-engineer relieved of duty. The Zonal Officer has also been suspended for failing to ensure proper coordination. “The zonal officer has been suspended because he should have seen overall coordination,” Commissioner Kumar said.
“We have found a few chambers that intersect the distribution line. We are getting them diverted,” Kumar also noted.
Authorities have stepped up efforts to contain the crisis and avert further casualties. District Magistrate Shivam Verma said the response has been extensive, with around 149 people currently hospitalised and survey teams conducting door-to-door checks. By 31 December, 2025, nearly 2,700 houses had already been covered.
The survey has been extended to other surrounding areas in Indore to check the water quality and to know whether the contamination has spread beyond Bhagirathpura, which was the initial epicentre. for precaution, ASHA workers and Auxiliary Nurse Midwives are also distributing oral rehydration solution (ORS) to residents.
© 2024 Bennett, Coleman & Company Limited

