- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Measles Outbreak Update: Kentucky Confirms Its First Case
Credits: Canva
The latest state to join the measles outbreak, which has been ongoing for quite some time now in the United States, is Kentucky. It has declared the outbreak, and has been confirmed by the US Centers for Disease Control and Prevention (CDC) on Wednesday. So far, there has been a total of 1,267 confirmed cases of measles this year, nationwide.
For now, five active measles cases have been detected in Kentucky, and four of these are linked to the same outbreak.
"When there are measles outbreaks in other states and nearby countries, it is not surprising to see spread to Kentucky," Steven Stack, M.D., secretary of the Kentucky Cabinet for Health and Family Services, said in a statement. "Measles can be very serious, but it is avoidable through vaccination. We urge all parents to have their children vaccinated to ensure they are protected from preventable diseases like measles."
Also Read: This Year, Doctor's Day Reminded That Doctors Too Are Humans And Can Get Emotionally Exhausted
Measles: How Many Outbreaks And How Many Cases?
The CDC defines an outbreak as at least three related cases, and so far in 2025, 27 such outbreaks have been reported. Of them, 88% of the confirmed cases, which means 1,115 cases out of 1,267, are linked with the ongoing outbreak. In 2024, a total of 285 measles cases were reported by 33 jurisdictions, resulting in 16 outbreaks.
Since the late winter of 2024 till spring of 2025, Texas witnessed the country's largest outbreak, and three additional cases were reported this week. The case count in Texas now totals 753 since late January.
Last week, the New Mexico Department of Health reported five measles cases at the Luna County Detention Center in Deming. Officials are currently determining the vaccination status of people being held at the facility.
"The cases at Luna County Detention Center are a stark reminder that the measles outbreak in New Mexico is not over," Chad Smelser, M.D., a medical epidemiologist with the New Mexico Department of Health, said in a statement. "We urge everyone in New Mexico, especially Luna County residents, to ensure that they are fully vaccinated against measles."
So far, from the measles cases in Texas, New Mexico and Oklahoma, three deaths have been reported. Among them, two were elementary school-aged children from the West Texas epicenter, and one was an adult in New Mexico. All of them were unvaccinated.
What Vaccine Must One Take To Prevent Measles?
The CDC recommends two doses of the MMR vaccine as the "best way to protect against measles, mumps, and rubella". For children, it recommends two doses of MMRV.
The MMR vaccine is a combination of measles, mumps, and rubella vaccines, while the MMRV is a combination of measles, mumps, rubella, and varicella (chickenpox) vaccines.
In the US, two MMR vaccines are available for use, including M-M-R II, and PRIORIX. For MMRV, the vaccine is only licensed for children who are 12 months through 12 years of age. The first dose is usually administered between the ages of 12 to 15 months, while the second dose is administered between the ages of 4 to 6.
For older children, adolescents and adults, the two doses of MMR vaccines should be separated by at least 28 days.
What Is Measles?
CDC notes that it is a highly contagious virus, which means if one person has it, up to 9 out of 10 people nearby will also become infected. As per the Mayo Clinic, measles is caused by the measles virus, which can spread through an infected person's cough, sneeze, or even during conversations.
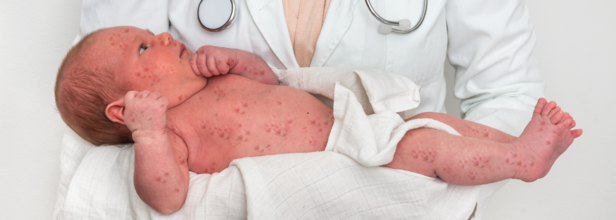
Measles: Signs And Symptoms
Measles symptoms appear 7 to 14 days after contact with the virus. Common measles symptoms include:
- High fever (may spike to more than 104° F)
- Cough
- Runny nose (coryza)
- Red, watery eyes (conjunctivitis)
- Rash
Jesy Nelson’s Fiancé Writes A Heartbreaking Poem As A Tribute After Twins’ SMA Diagnosis
Credits: Instagram
Jesy Nelson, former Little Mix singer, 34, and her fiancé, Zion Foster welcomed their twins, Ocean Jade and Story Monroe Nelson-Foster were devastated when the doctors broke the news that "they are probably never going to be able to walk, they probably will never regain their neck strength, so they will be disabled". Both the twins were diagnosed with SMA-1, a rare disease, known as the spinal muscular atrophy type 1.
Zion, 27, shared a poem for his eight-month-old warrior little girls. The Sun reported, he said: "They said it’s unlikely you’ll walk, you may not be able to talk, probably won’t be able to hold your head up, that’s what me and Jesy heard – SMA Type 1.
And it became so clear, doctors only go near what they can measure, so what’s certain?
I watch your smiles like sunsets, not promised, but real. I listen to you babble the sweetest melodies, in the moment it makes me wonder, if I keep telling you who I want you to be, what I want you to do, what I expect from you, am I loving you, or am I loving my fear?
If I take you for how God knitted you, just as you are, nothing removed, am I loving you? Am I accepting you?
Story, is your heart okay? Ocean, how’s your mind? I hear strength in your lungs every time you cry, two little warrior girls who already know how to fight.
Honestly, my worry isn’t the milestones, isn’t forcing life to live a different way. My worry is quieter than that, deeper. It’s about accepting you, loving you for who you are right now, without conditions.
No matter what tomorrow brings, and no matter what yesterday was."
What Treatment Jesy Nelson's Daughter Are Undergoing?
Jesy said, "They have had their treatment, thank God. A one-off infusion. That puts the gene back in their body that they don't have. It stops the muscles still working from dying. Any that have gone you can't regain them back."
While she does not reveal the name of the treatment, it is a single dose gene treatment, where patients receive an intravenous adeno-associated virus stereotype 9 carrying SMN complementary DNA encoding the missing SMN protein, as mentions a 2017 study published in The New England Journal of Medicine, or the Zolgensma, a prescribed gene therapy used to treat children less than 2 years old with SMA.
“Now it’s down to constant physio. We’ve been told they’ll probably never walk or regain their neck strength. They’ll probably be in wheelchairs.”
Jesy Nelson Twins: What Is SMA-1?
SMA-1. a rare disease, known as the spinal muscular atrophy type 1 or the Werdnig-Hoffmann disease is when the muscle weakness appears at birth or within the first six months. This rare condition prevents infants from sitting unassisted and causing severe breathing, swallowing, and sucking difficulties, leading to a poor prognosis without aggressive support. This condition has impacted the twin babies of the former Little Mix singer Jesy Nelson. Her twin babies may never be able to walk. However, she said that her babies will "fight all the odds" after they were being diagnosed with such a rare genetic condition.
Nelson said that there could be some common signs to look out for, which includes floppiness, inability to hold yourself up without support, a "frog-like" positioning of the legs without much movement, and rapid breathing in the tummy.
"If anyone is watching this video and they think they see these signs in their child, then please, please take your child to the doctor, to the hospital, because time is of the essence, and your child will need treatment. And the quicker you get this, the better their life will be," she added.
Flu Season Update: CDC Reports Declining Cases Across The US

Credits: Canva
Flu cases are starting to fall, yet experts caution that the United States still faces risks. In the week ending January 10, fifteen more children died from the flu, bringing the total pediatric deaths this season to 32, as per NBC News.
Flu Season: Latest CDC Data
On Friday, the Centers for Disease Control and Prevention (CDC) reported an 18% drop in confirmed flu cases compared with the previous week. Visits to doctors for respiratory illnesses decreased by more than 5%, and hospitalization rates fell by nearly 55%. Influenza-related deaths, however, rose by 2%.
So far this flu season, the CDC estimates that 18 million people have been infected, including 230,000 hospitalizations and 9,300 deaths.
“It seems like there is some cautious good news that cases are declining,” said Jennifer Nuzzo, director of the Pandemic Center at Brown University School of Public Health. “But I’m going to put a giant asterisk on this because that does not mean the worst is behind us.”
Last year, flu cases dipped around this time before climbing again in early February.
Flu Season: State-by-State Variation
CDC data reflect a national trend, but not all states have necessarily reached their peak. “We are not going to all experience this at the same time,” said Beth Carlton, a public health professor at the University of Colorado, as per NBC News. “Nationwide, the trend is downward, but different states and communities may see spikes as the virus spreads.”
Flu often appears first in densely populated areas like New York City before moving to rural regions, but the virus can behave unpredictably.
Flu Season: Impact on Schools
Although flu cases may be falling overall, other winter illnesses such as norovirus, Covid, and strep throat are still causing school closures in states including Arkansas, Kansas, Kentucky, Tennessee, and West Virginia.
High flu activity continues in Idaho, New Mexico, New York, and parts of Appalachia, while Montana, South Dakota, Vermont, and Wyoming report lower case numbers.
“The number of people hospitalized for influenza around New Year’s was extremely high—the second highest in the past decade, with last year being the highest,” Carlton said.
Flu Season: A Difficult Flu Season
This year’s severe flu season is driven by a heavily mutated strain of influenza A called H3N2 subclade K. Its mutations make it less similar to the strain used in this year’s vaccine. Influenza-like illnesses, including RSV and Covid, are also unusually high, Nuzzo said.
“Typically these viruses peak at different times, but this year they are peaking together, making the season particularly harsh,” she noted.
While there were concerns that the vaccine would be less effective against subclade K, recent research shows the current flu shot still offers protection, particularly against hospitalization. The vaccine covers three strains: H1N1, H3N2, and one B strain.
Flu Season: Pediatric Risk
As per NBC News, last year marked the deadliest flu season for children since the CDC began tracking pediatric deaths, with 289 children dying—more than during the 2009 H1N1 pandemic.
“That double peak last season clearly had consequences,” Nuzzo said. “Any decline this season is welcome, but we can’t assume the worst is over.”
Among children eligible for the flu shot whose vaccination status was known, 90% of pediatric deaths occurred in unvaccinated kids.
Following recent CDC guidance, flu shots are no longer recommended for all children, a change from the previous advice that everyone six months and older should be vaccinated annually.
Anktiva Approved In Saudi Arabia As World’s First Lung Cancer Therapy — All You Need to Know
Credits: Canva
The Saudi Arabian Food and Drug Authority has cleared Anktiva, an IL-15–based immunotherapy created by billionaire physician-scientist Patrick Soon-Shiong and his biotech firm ImmunityBio, for the treatment of bladder cancer and lung cancer. This marks the first time the therapy has received national regulatory approval outside the United States.
The move signals an important global step for Anktiva, which currently holds a limited approval from the U.S. Food and Drug Administration. In the U.S., the drug is authorised only for patients with BCG-unresponsive, non–muscle-invasive bladder cancer (NMIBC) that includes carcinoma in situ. American regulators have so far resisted expanding its use to other bladder cancer subtypes. Saudi regulators, however, have adopted a broader stance, approving Anktiva for two cancer types under their domestic regulatory system.
What Is Anktiva?
Anktiva is an interleukin-15 receptor agonist designed to stimulate the body’s own immune defences. It works by activating and expanding natural killer (NK) cells and memory CD8⁺ T cells, which play a key role in immune surveillance. Unlike chemotherapy or gene-based treatments, Anktiva does not attack tumour cells directly. Instead, it boosts existing immune pathways to help the body recognise and destroy cancer cells.
ImmunityBio describes Anktiva as “the first FDA-approved immunotherapy that activates what’s called a natural killer cell to target and kill non-muscle-invasive bladder cancer cells.” In clinical practice, the drug is used alongside BCG (Bacillus Calmette-Guérin) in patients whose NMIBC has not responded to BCG alone. It is administered directly into the bladder through a catheter, followed by a structured maintenance schedule
Clinical Evidence Supporting FDA Approval in NMIBC
The FDA’s approval, issued on April 22, 2024, was based on results from a single-arm clinical study involving 77 patients with BCG-unresponsive stage 0 NMIBC. Participants received intravesical Anktiva combined with BCG, with maintenance therapy continuing for as long as 37 months.
The main efficacy results were as follows:
- Complete response rate: 62%
Durability:
- 58% of patients who responded remained disease-free for at least 12 months
- 40% stayed disease-free for 24 months or longer
For patients facing the prospect of radical cystectomy as the only curative option, these results were considered clinically significant. Still, the absence of a randomised control group has remained a point of contention among regulators and experts.
Regulatory Tension in the United States
Although the FDA approved Anktiva for NMIBC cases involving carcinoma in situ, it declined to extend the indication to patients with papillary-only disease. ImmunityBio pushed back against the decision, arguing that the same clinical data had already been deemed sufficient to support approval in a closely related patient group.
Rachel Sherman, MD, former principal deputy commissioner of the FDA, publicly criticised the agency’s stance, saying: “it is incomprehensible to me that the FDA refuses to file a supplemental BLA, stating the study is not sufficient to support a regulatory review, when it has already approved a product based on that very same study in essentially the same indication and population.”
The FDA has also expressed concerns about how the drug has been marketed. It issued a warning letter to ImmunityBio over promotional materials that cited survival benefits and cystectomy-avoidance rates not supported by robust evidence.
Saudi regulators, by contrast, have taken a more permissive view, approving Anktiva for both bladder and lung cancer and highlighting a willingness to act in areas of high unmet medical need.
With this decision, Saudi Arabia becomes the first country to approve Anktiva beyond NMIBC, potentially placing itself at the forefront of evaluating the therapy’s wider role across multiple cancer types.
© 2024 Bennett, Coleman & Company Limited

