- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Medical Cannabis May Help Fight Cancer, Largest-Ever Study Shows Potential To Treat Symptoms

Credits: Canva
The largest-ever scientific study on cannabis and cancer has shown strong evidence that medical cannabis can do more than treat symptoms — it could actually fight the disease itself. The research, published in Frontiers in Oncology, provides a robust and data-heavy analysis that brings much-needed clarity to a very contentious issue.
Led by Ryan Castle, Research Director of the Whole Health Oncology Institute, this revolutionary analysis converges more than 10,000 studies — the largest such inquiry into medical cannabis and cancer to date.
Cannabis has been in the middle of medical controversies and legislative wars for a long time. Traditionally, the Schedule I status of cannabis under federal law has limited high-quality human clinical studies, preventing the medical community from reaching a consensus. Castle and his colleagues aimed to change that.
Our aim was to establish the scientific consensus on the issue of medical cannabis, an area that has been long dominated by a war of cherrypicked studies," Castle said.
In order to transcend prejudice, Castle's team took the large-scale, inclusive approach fuelled by AI and sentiment analysis — a process widely employed by natural language processing to determine whether written material portrays a positive, neutral, or negative sentiment. Here, AI analyzed thousands of abstracts and conclusions drawn in scientific literature to determine whether each one stated agreement, neutrality, or doubt in cannabis's applicability to the treatment of cancer and symptom alleviation.
The result? An overwhelming majority of studies presented a positive view, indicating medical cannabis holds therapeutic value not just for symptom relief — such as reducing inflammation and nausea or boosting appetite — but potentially for accelerating apoptosis, the death of cancer cells.
Castle's group examined over 10 times the amount of research examined in any other meta-analysis. Their report stated that roughly 55% of studies indicated a positive relationship between medical cannabis and favorable cancer outcomes with only a few percent reporting adverse effects or none at all.
That percentage — 55% — may seem humble, but with the sheer scale of the data and the scientific conservative tradition in this area, it's telling. "This level of statistical consensus is precisely what we required to start thinking of cannabis as more than an edgy cure-all," Castle wrote.
In addition, the National Cancer Institute (NCI) revealed that 20% to 40% of patients with cancer currently use marijuana products to deal with side effects such as constant pain, chemotherapy-related nausea and vomiting, and sleeplessness. Still, investigations had fallen behind trend usage rates based on government policy and availability of funds in the past.
It is valuable to place the results of this mega-study in the context of the overall body of cannabis research. Much of what is published is derived from in vitro (test tube) experiments or animal models and not human trials. However, several of these studies have promising results: compounds found in cannabis — particularly cannabinoids such as delta-9 THC, CBD, and CBG — have been shown to inhibit cancer growth, prevent metastasis, and cause cancer cell death in laboratory experiments.
A 2023 Discover Oncology study supported this perspective, reaching the conclusion that multiple cannabinoids reveal "promising potential as anticancer agents by multiple mechanisms." These include curbing tumor expansion, inhibiting cancer cell invasion, and cutting inflammation — which is a documented driver of cancer growth.
In addition, more recent studies have discovered unforeseen advantages in cancer patients who use cannabis. A University of Colorado study reported that patients who consumed marijuana products from licensed dispensaries for a two-week period reported better thinking and cognition, contrary to previous concerns that cannabis would impair mental sharpness in chronic users.
Even with all the encouraging results, Castle and other specialists advise not to consider cannabis a panacea. The available evidence does not indicate that medical cannabis by itself can heal cancer. Rather, its actual strength is in integrative oncology — as an adjunct therapy in addition to standard therapies such as chemotherapy, radiation, and immunotherapy.
This is in agreement with results of a 2019 literature review, which noted that cannabis potentially slows cancer growth and aids improved treatment outcomes, although its effectiveness is highly variable based on cancer type, formulation of cannabinoids, and dose.
One of the significant challenges that persist in holding back development in this arena is the federal classification of cannabis as a Schedule I drug. According to existing U.S. policy, cannabis is listed alongside drugs such as heroin — a designation that presents many legal and bureaucratic hurdles to researchers and healthcare professionals.
In addition, in the Trump era, the National Cancer Institute highlighted marijuana as one of almost two dozen "controversial or high-profile issues" that needed extra clearance prior to publication or research sharing. This culture of fear and repression has only held back the much-needed research into medical cannabis's complete potential.
The biggest-ever analysis of medical cannabis and cancer isn't asserting to have discovered a cure but what they have discovered is a mounting scientific concurrence that medical cannabis should be included in mainstream discussions of cancer treatment. From reducing side effects to having the potential to interfere with cancer cell life cycles, cannabis might have more than just palliation to provide — it might have clinical benefit.
New 'Frankenstein' Covid Variant, Now Called The 'Halloween Monster In The Air' Is Spreading Faster Than Ever, What Makes It So Unique?
Credits: Canva
The new 'Frankenstein' variant of COVID-19 is spreading rapidly. It has been largely ignored, however, this new SARS-CoV-2 variant is causing high levels of sickness absence in nurseries, schools and care facilities and will also increase the number of long COVID patients.
It is the same as the Stratus variant, scientifically known as XFG. It is nicknamed as 'Frankenstein'. Many are also calling this variant the "Halloween Monster in the Air", as it has led to a 37% spike in hospital visits in France.
What Is Happening In The World?
In a very short time, the "Stratus" variant has become the dominant strain. In Germany, it has accounted for 84% of identified SARS-CoV-2 variants at the beginning of October 2025. This variant is also the dominant one in Austria and Switzerland, making up for about 80% of viral load in wastewater.
As per the Robert Koch Institute (RKI), it has also led to rise in acute respiratory illnesses since September. The Frankenstein virus has affected people across ages.
Why Is It Called 'Frankenstein' Variant?
The new variant gets its name 'Frankenstein' due to its genetic fusion of components from different virus types, which makes it an accurate description of a genetic monster. The new 'Frankenstein' Covid variant is a recombinant variant formed from the Omicron lineages LF.7 and LP.8.1.2. The fusion has led to a more resistant and "fitter" strain.
As per the World Health Organization (WHO), the rise in Covid-19 cases are also associated with the same variant. It has been classified by the WHO as a variant under monitoring since June 25, 2025. The evidence while show that the additional public health risk is low worldwide, the mutation makes it more contagious, thus the growing number of cases.
What Makes The New 'Frankenstein' Covid Variant So Unique?
The symptoms, while some of them are quite similar to that of we know from COVID-19, there are some unusual symptoms too, which were often associated with a common cold:
- Sore throat
- Runny nose and congestion
- Dry cough
- Fatigue and muscle pain
- Mild fever
- Loss of appetite
- Shortness of breath
- Chest pain
- High fever in people with lower or weakened immunity system
What Are The Ways You Could Prevent The New 'Frankenstein' Covid Variant?
At present, there is no specific treatment available for the Frankenstein variant. The approach remains similar to that for other COVID-19 strains, focusing on managing symptoms according to how the illness progresses.
Vaccination: Current vaccines are believed to help reduce the risk of severe illness and hospitalization. While their effectiveness may slightly decrease against new variants, they continue to play a crucial role in prevention.
Supportive care: Simple measures such as taking fever-reducing medications, staying well-hydrated, getting enough rest, and using cough suppressants can help ease symptoms.
Preventive practices: Wearing a mask, maintaining proper hand hygiene, avoiding crowded spaces, and keeping a safe distance from others remain key protective steps.
As research on the Frankenstein variant continues, individual protective measures are vital. Because the illness can affect each person differently, it’s important to consult a healthcare professional if symptoms appear.
Delhi Pollution Left People Gasping For Air, But There Are Ways To Stay Safe According To Doctor
Credits: Canva and AQIcn.org
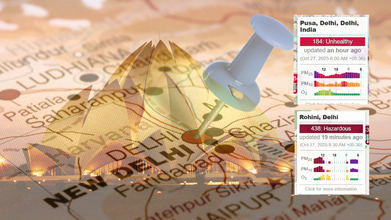
After some days of relief, on Sunday, Delhi again woke up to a thick layer of smog, with pollution levels rising up to 'very poor' category. The centre's early warning system (EWS) forecast that air quality index (AQI) will further deteriorate.
On Sunday, by 7pm, Delhi's AQI was at 299, just below 'very poor' level on the index. Anand Vihar remained at severe levels with the AQI logged at 421 at 11am, by evening, it rose to 428. Wazirpur also slipped into the 'severe' category with an AQI of 408.
As Delhi continues to struggle with smog-filled skies, and thick layer of smoke to breathe through, an earlier video by Dr Divya Prakash, Consultant Physician at Yashoda Hospital on tips to stay safe amid Delhi pollution is making rounds.
What Does The Doctor Say?
Dr Prakash points out that the moment the index goes above 50, the air starts to become unhealthy for us, however, in Delhi, we already see the AQI levels crossing the 400 threshold.
"The main problem is with our lungs due to increasing air pollution. It also indirectly affects our heart and brain. So, how can we save ourselves from this?" He shares that the best way is to use public transports and electric vehicles. He also recommends wearing a mask whenever anyone goes out and urges people to avoid going out at peak hours.
"Eat healthy food, avoid burning wood or construction work around the house and do use your air purifier at home, especially for children and elderly and those who already have lung or other heart problems," he says.
How Poor AQI Impacts Our Lungs?
Air pollution comprises of tiny pollutants and particles called the PM2.5 and PM10, that reach deep into our lungs. These particles, though tiny, are able to inflame the airways and cause breathlessness, wheezing, and repeated coughing. This can further cause healthy adults to suffer from throat irritation, headaches, and fatigue if they are exposed for a long time.
Since children inhale more air per kilogram of body weight than adults, they are at more risk of being harmed by the pollutants. Furthermore, they also have a weaker immunity, which puts them at more risk of such complications.
The World Health Organization (WHO) also notes that not just lungs, but almost every organ in the body can be impacted by air pollution. Thanks to the small size of the pollutants, they can penetrate into the bloodstream via lungs and then circulate throughout the body and could lead to systemic inflammation and carcinogenicity.
What Diseases Could Air Pollution Cause?
Apart from the respiratory diseases like asthma, shortness of breath, COPD, WHO notes that air pollution could is a risk for all-cause mortality as well as diseases like:
- Stroke
- Ischaemic heart disease
- Lung cancer
- Pneumonia
- Cataract
There are evidence that support the link between air pollution exposure and adverse pregnancy outcomes, including low birth weight of the child, small for gestational age, and other cancers, diabetes, and cognitive impairment and neurological disorders in the child.
How Can You Read AQI?
As per the Central Pollution Control Board, here's how the data on AQI can be interpreted
- 0-50 is considered ‘good’
- 51-100 is considered ‘satisfactory’
- 101-200 is considered ‘moderate’
- 201-300 is considered ‘poor’
- 301-400 is considered ‘very poor’
- 401-500 is considered ‘severe’
Delhi’s Air Quality Slips Back to ‘Very Poor’; Toxic Air Could Cause Acute Health Effects, Warns AIIMS Doctor

Credits: Canva
Delhi witnessed a slight improvement in its Air Quality Index (AQI) for a couple of days before it again slipped back to 'very poor' category. On Sunday morning, some areas in fact reached 'severe' category on the AQI.
While pollution spiked during Diwali, on Friday and Saturday, Delhi's AQI was recorded at 275 and 292 respectively, which has placed the air quality category in 'poor'.
However, on Sunday morning, Delhiites woke up to 'very poor' air quality, thanks to the thick layer of smog. The AQI was recorded well above 300 in most areas. Visuals too show low visibility in many areas of the national capital.
What Does The Expert Say About The Air Quality?
On Sunday, as of 7am, Delhi's Anand Vihar area recorded an AQI of 430, which has placed it under the 'severe' category. Wazirpur also recorded an AQI of 403, further placing it under the 'severe' category. Speaking to ANI, former AIIMS Director Dr Randeep Guleria urged people to use preventative measures in order to minimize their exposure to the polluted air. He also warned people of the health implications which could be the result of deteriorating air quality.
"The current high levels of air pollution, indicated by poor AQI, are leading to acute health effects, particularly among individuals with underlying heart or lung conditions, the elderly, and young children. These groups are experiencing increased chest discomfort, breathing difficulty, cough, and worsening of pre-existing conditions like asthma and COPD," Dr Guleria told ANI, on Friday.
"Even healthy individuals are reporting symptoms such as nasal stuffiness, throat pain, chest tightness, and coughing. The inflammation and narrowing of airways caused by pollutants are contributing to these issues. Additionally, the use of crackers, despite permissions for 'green crackers,' has exacerbated air pollution," he added.
What Preventative Measures Should One Use?
The best way to protect yourself is by limiting your outdoor timings, especially during the early morning hours and at the night. Smog is at its lowest during the afternoon. Furthermore, using an N-95 respirator could protect you from the minute pollutant particles in the air.
N-95 is a respiratory protective device designed to achieve a very close facial fit and very efficient filtration of airborne particles, notes the FDA. This is what makes it fit for use during high levels of pollution.
The edges of N-95 are designed to form a seal around the nose and mouth. Some models even have exhalation valves that can make breathing out easier and help reduce the heat build-up.
As per a 2021 study published in the Indian Journal of Radiology and Imaging, the N-95 mask could block "at least 95% of very small test particles".
Read More: Which Mask To Wear For Best Protection?
What Does AQI Mean?
As per the Central Pollution Control Board, here's how the data on AQI can be interpreted
- 0-50 is considered ‘good’
- 51-100 is considered ‘satisfactory’
- 101-200 is considered ‘moderate’
- 201-300 is considered ‘poor’
- 301-400 is considered ‘very poor’
- 401-500 is considered ‘severe’
Delhi AQI as of 7am
Alipur, Delhi (DPCC) - 309.00
Anand Vihar, Delhi (DPCC) - 430.00
Ashok Vihar, Delhi (DPCC) - 369.00
Aya Nagar, Delhi (IMD) - 272.00
Bawana, Delhi (DPCC) - 390.00
Burari Crossing, Delhi (IMD) - 344.00
CRRI Mathura Road, Delhi (IMD) - 330.00
Chandni Chowk, Delhi (IITM) - 376.00
DTU, Delhi (CPCB) - 266.00
Dr. Karni Singh Shooting Range, Delhi (DPCC) - 317.00
Dwarka-Sector 8, Delhi (DPCC) - 301.00
IGI Airport (T3), Delhi (IMD) - 269.00
IHBAS, Dilshad Garden, Delhi (CPCB) - 310.00
ITO, Delhi (CPCB) - 329.00
Jahangirpuri, Delhi (DPCC) - 370.00
Jawaharlal Nehru Stadium, Delhi (DPCC) - 304.00
© 2024 Bennett, Coleman & Company Limited

