- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Mom Gives Birth To Extremely Rare “MoMo” Twins In Back-To-Back Pregnancies Within 13 Months

Image Credits: Britney Alba
In an extraordinary and rare medical case, mother Britney Alba of Tuscaloosa, Alabama, delivered two pairs of identical twins over a mere 13 months. Such a feat is mind-boggling, much less likely to happen than winning a jackpot or being hit by lightning. Alba had her second set of identical twins at the University of Alabama at Birmingham (UAB) Women and Infants Center, which is a case that has captivated medical personnel as well as the general public.
Alba's second pair of twins were not only identical but also monoamniotic-monochorionic (MoMo) twins, one of the most unusual forms of twin pregnancies. MoMo twins have the same placenta, amniotic sac, and fluid, which makes them extremely vulnerable to life-threatening complications. This condition is extremely rare, found in fewer than 1% of all births in the United States.
The four-year-old mother was at first shocked upon being told she was pregnant with twins again, just six months after the birth of her first twins, Luka and Levi. As at first thrilled, she and her husband Frankie, later had to confront the harsh reality of the risks involved in carrying MoMo twins.
Medical Risks Involved in MoMo Pregnancies
According to Dr. Rachel Sinkey, a UAB Marnix E. Heersink School of Medicine Department of Obstetrics and Gynecology assistant professor, MoMo twins are among the highest-risk twin pregnancies. "They have everything in common except umbilical cords, which could easily get tangled up and cause fetal distress or a stillbirth," Dr. Sinkey said.
Because of these dangers, Alba's pregnancy was intensively monitored. Doctors in the Division of Maternal-Fetal Medicine at UAB suggested she be placed on a high-risk obstetrics unit during weeks 24 through 28 of gestation. She eventually went in at 25 weeks, where she underwent around-the-clock monitoring to reduce the risk of complications of umbilical cord entanglement and others.
A High-Stakes Delivery and Postnatal Care
Due to the increased risk of MoMo pregnancies, doctors take careful precautions to have the baby delivered as safely as possible. Following the national standard of care, Alba had a planned cesarean section at 32 weeks, since after this week, waiting longer could further enhance the risk of complications in the umbilical cord.
The second pair of twins, Lynlee and Lydia, were delivered on October 25, 2022. Immediately after they were born, the twins were sent to the neonatal intensive care unit (NICU) to receive specific attention. The twins stayed for some weeks in the newborn nursery before being discharged on December 7, marking the culmination of a trying yet successful experience for Alba and her family.
Comparing Alba's Two Twin Pregnancies
Alba's initial set of twins, Luka and Levi, were monochorionic-diamniotic (MCDA) twins, so they had the same placenta but separate amniotic sacs. While this form of twin pregnancy has risks, they are higher with MoMo twins and it happens at a frequency of three or four in every 1,000 live births.
MCDA twins, such as Luka and Levi, are at risk for twin-to-twin transfusion syndrome (TTTS), where one twin gets dramatically more blood and nourishment than the other. Thankfully, both Luka and Levi were delivered without issues. But the MoMo twin pregnancy of Lynlee and Lydia was even riskier because the babies lacked a separating membrane, so there was greater risk for life-threatening umbilical cord intersections.
Management of MoMo Twin Pregnancies
MoMo pregnancies need careful monitoring to maximize outcomes. The routine management is frequent ultrasound, inpatient care in the latter part of pregnancy, and scheduled early delivery by cesarean section. This reduces significantly the risk of stillbirth and other complications.
Dr. Sinkey stressed the significance of multidisciplinary care in treating such high-risk pregnancies. "The team care at UAB, including maternal-fetal experts, genetic counselors, nurses, and the NICU team, was instrumental in achieving a good outcome for Britney and her daughters," she stated.
Though MoMo twins experience tremendous challenges prior to birth, post-birth care is just as essential. Because of their premature delivery, Lynlee and Lydia needed intensive care in the NICU, where they were under constant surveillance for breathing, feeding, and general growth. The twins were discharged in December 2022, which was a welcome end to their amazing adventure.
The case of Alba illustrates the value of specialized medical care during high-risk pregnancies. Though weighed heavily against her, the experience of her family is one of tribute to medicine's progress, caring healthcare providers, and a mother's strength.
MoMo twin gestations are a field of significant interest in maternal-fetal medicine because they are extremely rare and so high-risk. Alba's experience of back-to-back twin pregnancies, where the second is MoMo twins, is among the most rare documented cases.
From disbelief at first about learning she was pregnant with twins once again to the monitoring and eventual successful birth, her story highlights the power of medical expertise and parental unyielding strength. Her account will be the subject of study for years to come as a rare but motivational instance of beating the odds in maternal health.
Michael Clarke Reveals Skin Cancer Surgery, Sparking Urgent Reminder: How To Spot The Signs Early?

Credits: Instagram/Michael Clarke
Former Australian cricket captain Michael Clarke has once again faced a health struggle that affects millions around the globe, skin cancer. Clarke, 44, posted on social media that he recently had surgery to have a suspicious growth removed from his nose. His post was not just about healing, but also an appeal: get your skin checked.
This reminder from a sports legend underscores a pressing global health issue. Skin cancer is the most common cancer diagnosed in the United States, Australia, and many other parts of the world. The good news is that when detected early, most cases are highly treatable but that early detection depends on awareness knowing what skin cancer looks like and when to seek medical help.
Clarke's recent diagnosis is not his initial experience with skin cancer. In 2006, doctors operated on him to remove suspicious marks on his face and chest. They removed them surgically before they were able to do further damage. Again in 2019, he had surgery to have cancerous tumors removed from his forehead.
Now, in 2025, his post-surgery update again emphasizes how skin cancer can be a recurring aspect for those at risk. Clarke had penned, "Skin cancer is real! Particularly in Australia. Another one removed from my nose today. A friendly reminder to have your skin checked. Prevention is better than the cure but in my case, regular check-ups and early detection is the key."

Australia has some of the highest rates of skin cancer on the planet because of high levels of UV radiation. However, Clarke's experience sounds off far beyond his native soil. In the United States alone, more than 5 million instances of skin cancer are treated annually, the American Cancer Society says.

What Is Skin Cancer?
Skin cancer occurs when skin cells grow out of control, penetrating surrounding tissue and occasionally spreading to other organs. The sun's ultraviolet (UV) light is the primary cause, though indoor tanning is also a culprit. There are three types of skin cancer with varying risks and appearances:
Basal Cell Carcinoma (BCC): The most frequent one. BCC typically appears as a flesh-colored bump, pearl-like growth, or pinkish lesion. It usually develops on sun-exposed sites such as the face, neck, and arms. Although it does not spread much, if left untreated, it can produce extensive local destruction.
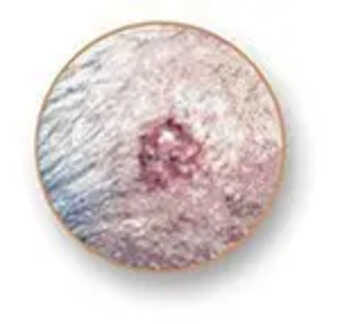
Squamous Cell Carcinoma (SCC): The second most frequent type. SCC can be a hard red bump, scaly area, or sore that closes up and reopens. It often occurs on sun-exposed areas like the ears, lips, and face. If left alone, SCC can grow and invade deeper tissues.

Melanoma: The most life-threatening form. Melanoma can develop in a pre-existing mole or as a new dark spot that appears different from surrounding markings. It grows more quickly than other skin cancers, so catching it early is paramount.

SCC can develop from a precancerous skin growth: Actinic keratoses are primarily caused by cumulative sun exposure and tend to develop on areas of the body that get the most sunlight, including the face, scalp, neck, hands, and forearms. People with fair skin are more likely to develop these patches, but they can occur in anyone with a history of significant sun exposure.

What Does Skin Cancer Look Like?
One of the difficulties with skin cancer is that its early signs are simple to overlook or assume to be harmless. However, being in a position to recognize these alterations can be the difference between life and death. General warning signals are:
- A new growth that looks like a mole, bump, or scab.
- An open sore that does not heal.
- A patch of skin that is rough or scaly.
- Alterations in a mole getting larger, darker, or becoming irregular in shape.
- Persistent itching or pain around a skin spot.
Doctors recommend following the ABCDE rule for melanoma:
Asymmetry: One half doesn’t match the other.
Border: Edges are irregular or blurred.
Color: Uneven colors—brown, black, red, or white patches.
Diameter: Larger than 6mm (about the size of a pencil eraser).
Evolving: Any mole or spot that changes in shape, size, or symptoms.
Where Skin Cancer Can Appear?
Most individuals think that skin cancer only occurs on sun-exposed skin. Although it is correct that the scalp, face, neck, arms, and hands are the most susceptible, cancers can occur on less visible locations:
- Under the toenail or fingernail.
- On the palms or soles of the feet.
- Around the genitals.
- On the back, which individuals might seldom examine themselves.
This is particularly the case for individuals with darker skin. In these individuals, melanoma and other types tend to appear on less sun-exposed areas, i.e., under nails or on the feet.
Who Is at Greatest Risk?
Anyone may get skin cancer, but certain individuals have a higher risk based on specific characteristics. People with fair skin, light hair, and light eyes are at greater risk, as are those with past histories of severe sunburns, particularly blistering. Frequent sun exposure with poor protection and tanning bed use also increase the risk of skin cancer. An inheritance pattern of the illness or compromised immune system further increases the risk. Michael Clarke's years of cricket playing in the strong Australian sunlight emphasize how long-term exposure to the sun can dramatically raise the risk of skin cancer.
Is Skin Cancer Preventable?
While you cannot alter your genes or your skin type, you can lower your risk considerably:
- Use sunscreen daily with SPF 30 or better. Apply every two hours when you're outside.
- Wear protective gear, wide-brimmed hats, sunglasses, and long sleeves.
- Seek shade between 10 a.m. and 4 p.m. when the sun's rays are strongest.
- Steer clear of tanning beds, which release cancer-causing UV radiation.
- Have your skin checked regularly, both at home and by a dermatologist.
Clarke's focus on early diagnosis is echoed in medical guidance: the sooner you detect changes, the greater the potential for effective treatment.
Why Regular Checkups Are So Important?
Skin cancer often develops silently. A small bump today may not cause pain but could evolve into something dangerous over time. Clarke’s case illustrates why vigilance is critical even after previous treatments. Recurrence is possible, and only regular screenings can catch issues before they progress.
The American Academy of Dermatology recommends annual full-body skin checks for most adults, and more frequent exams for those with higher risk factors.
Michael Clarke's candor regarding his diagnosis puts a spotlight on a public health problem that affects the entire world. His tale is not merely that of an athlete undergoing another surgery it is a reminder to all, wherever they may be in the world, that skin cancer exists, is prevalent, and is frequently preventable.
Legionnaires’ Outbreak Canada: 94 Sick So Far, Ontario Food Manufacturer's Cooling Tower Could Be Source Of The Deadly Disease

Credits: Canva
Legionnaires Outbreak: Public health officials in London, Ontario, have redeclared a Legionnaires’ disease outbreak after 25 new cases emerged, weeks after declaring the situation under control. The Middlesex-London Health Unit (MLHU) confirmed Tuesday that testing has identified a likely source: cooling towers at Sofina Foods Inc., a large meat-processing facility on Trafalgar Street.
Outbreak Declared Over, Then Returned
The outbreak was first detected in early July and by early August had led to 70 confirmed cases and three deaths. After three weeks without new infections, the MLHU declared the outbreak over on August 6. But within days, new patients began appearing.
As of this week, the caseload has climbed to 94, including 86 hospitalizations and four deaths. Six patients remain in hospital. Officials say the resurgence highlights how the bacteria can persist in contaminated environments despite earlier cleaning efforts.
“For several weeks, no additional illnesses were reported, and we were optimistic that remediation efforts had eliminated the bacteria. However, 25 more people have now become ill,” said Dr. Joanne Kearon, associate medical officer of health. “Fortunately, a likely source has now been identified.”
Read: Legionnaires Outbreak in New York: All That You Need To Know About The Disease
Cooling Towers Traced as the Source
Extensive environmental testing linked the outbreak strain of Legionella bacteria to Sofina Foods’ cooling towers. Samples taken in recent weeks matched the same bacterial subtype found in patient cases.
While nine different cooling towers across the city initially tested positive for live bacteria, the Sofina plant produced a definitive genetic match. Earlier testing at the site in 2024 and early 2025 had shown no match, but officials said the bacteria can survive in cooling towers and regrow under hot, humid conditions.
The health unit emphasized that food products from Sofina remain safe to consume, since Legionnaires’ disease spreads only through inhaling contaminated water droplets, not through eating or drinking.
Sofina Foods Responds
Sofina Foods, which employs hundreds of workers in London’s east end, said the new findings were “unexpected” given the company’s daily sanitation protocols and previous negative test results.
“We are deeply concerned with this new information and are continuing to investigate fully,” said Sharon Begley, Sofina’s chief safety officer. “Over and above our regular processes, we conducted further deep cleaning and disinfecting processes recommended by MLHU. The cooling tower is offline and will remain offline until these additional steps are completed.”
The company stressed its commitment to employee and community safety, noting it has been cooperating closely with public health authorities since the outbreak began.
How Legionnaires’ Spreads
Legionnaires’ disease is a severe form of pneumonia caused by Legionella bacteria. It is not transmitted from person to person. Instead, people get sick when they inhale mist or small droplets of water containing the bacteria, which can be carried by wind from sources such as cooling towers, hot tubs, and water systems.
Symptoms typically appear within 2 to 10 days of exposure and may include fever, chills, cough, muscle aches, and shortness of breath. Most exposed individuals do not get sick, but older adults, smokers, and people with weakened immune systems face greater risk of serious illness.
The current outbreak has been concentrated in a six-kilometre radius in London, with cases ranging from young adults to seniors. No children have been affected to date.
Also Read: Legionnaires' Outbreak Update: It Is No Longer 'Only A New York Problem'
More Cases Possible
Dr. Kearon warned that more cases could surface due to the bacteria’s incubation period. “Legionella has an incubation period of two to 10 days ... there may still be further cases in the next two weeks,” she said.
This year’s outbreak is already the largest in London’s recent history, surpassing a 2024 outbreak that caused 30 cases and two deaths. Officials say they are working with Sofina Foods and other operators to ensure thorough remediation and prevent further spread.
“The decision to reopen the outbreak reflects a rise in cases after several weeks without new cases, suggesting that the Legionella bacteria has re-emerged in the environment despite earlier remediation efforts,” MLHU said in a statement.
Eli Lilly Sends Weight-Loss Pill For Approval: Is Oral GLP-1 As Effective As The Injections?

Credits: Canva
Eli Lilly has taken a major step in the fight against obesity and diabetes. The company announced that its oral GLP-1 drug, orforglipron, successfully met the “primary and all key secondary endpoints” in a large clinical trial, paving the way for global regulatory submissions.
“With these positive data in hand, we are moving with urgency toward global regulatory submissions to potentially meet the needs of patients who are waiting,” said Kenneth Custer, Eli Lilly’s executive vice president and president of cardiometabolic health.
“If approved, we are ready to offer a convenient, once-daily pill that can be scaled globally, removing barriers and redefining how obesity is treated around the world.”
What Is Orforglipron?
Orforglipron is an investigational, non-peptide, once-daily small molecule GLP-1 receptor agonist. Unlike some oral medications that come with strict food or water restrictions, it can be taken at any time of day without such limitations.
Originally discovered by Japan’s Chugai Pharmaceutical and later licensed to Eli Lilly in 2018, orforglipron is now in Phase 3 trials. The company is testing it not just for weight management and type 2 diabetes but also for conditions linked to obesity, including obstructive sleep apnea and hypertension.
The ATTAIN-2 Trial Results
The company’s latest clinical trial, called ATTAIN-2, produced results that have drawn significant attention. At the highest dose: 36 mg daily for 72 weeks, participants saw an average weight loss of 22.9 pounds (10.5%), compared to just 5.1 pounds (2.2%) among those given a placebo.
The drug also delivered strong results for people with type 2 diabetes. Across doses, orforglipron reduced A1C levels by 1.3% to 1.8% from a baseline of 8.1%. Notably, 75% of participants who took the highest dose achieved an A1C of 6.5% or below, aligning with the American Diabetes Association’s definition of diabetes remission.
Beyond weight and blood sugar, orforglipron showed broader health benefits. The trial reported improvements in cardiovascular risk factors such as non-HDL cholesterol, systolic blood pressure, and triglyceride levels. In an exploratory analysis, the drug also cut high-sensitivity C-reactive protein, a key marker of inflammation, by about 50%.
Why An Oral Option Matters
Injectable GLP-1 receptor agonists like semaglutide and tirzepatide have already transformed obesity and diabetes care, but accessibility remains a hurdle. For many patients, injections are intimidating, inconvenient, or simply not practical in daily life.
That’s where an oral pill could be a game-changer. A once-daily tablet could remove psychological and logistical barriers, making it easier for patients to stay on treatment. And given the rising global burden of obesity, which significantly raises the risk of cardiovascular disease, stroke, and some cancers, the demand for more convenient treatment options has never been greater.
Pills vs. Injections: Which Works Better?
The big question now is whether oral GLP-1 drugs are as effective as their injectable counterparts.
A 2021 research review published in Springer Nature, offers some clues. After examining multiple studies, researchers concluded that oral semaglutide, a similar class of drug, provided “similar or better efficacy and similar tolerability” compared to injectable GLP-1 receptor agonists.
Also Read: How To Identify A Counterfeit Ozempic? Look For These Signs
In some cases, oral versions were found to be just as effective for weight loss and lowering A1C levels in people with type 2 diabetes. However, the review focused on patients already using insulin, which may have influenced outcomes. Experts emphasize that while results are encouraging, more research is still needed to directly compare oral and injectable versions in broader populations.
For now, injections remain the gold standard in clinical practice. Drugs like Wegovy and Mounjaro (both injectables) have shown weight reductions of 15% to 20% or more in major trials, higher than the 10.5% seen with orforglipron in ATTAIN-2. That said, the convenience of a pill could tip the balance for many patients, especially those reluctant to use injections long-term.
Eli Lilly’s oral GLP-1 drug is not yet approved, but the latest results highlight its potential to reshape treatment strategies for obesity.
© 2024 Bennett, Coleman & Company Limited

