- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
NHS Warns Against Omeprazole, One of the UK’s Most Prescribed Drugs, Know Why

Credits: Canva
Omeprazole is among the most frequently prescribed medicines in the UK. The latest NHS figures show that between 2022 and 2023, over 73 million prescriptions for proton pump inhibitors (PPIs), such as omeprazole, were dispensed in England alone. That means nearly 15% of the population may be taking these drugs.
PPIs are medications designed to reduce stomach acid production. They achieve this by blocking proton pumps, enzymes located in the lining of the stomach that are responsible for generating acid. By lowering acid levels, PPIs help manage conditions such as acid reflux and heartburn, which affect millions of people.
Also Read: Is Huntington’s Disease Genetic? Here’s Everything You Should Know
Conditions Managed by PPIs
The role of PPIs extends far beyond occasional heartburn. They are commonly prescribed to treat:
- Indigestion
- Healing stomach ulcers
- Gastro-oesophageal reflux disease (GORD/GERD)
- Acid-related inflammation of the food pipe
In addition, omeprazole and other PPIs are sometimes prescribed alongside antibiotics to treat Helicobacter pylori, a stomach infection that can lead to ulcers if left untreated.
Commonly Prescribed PPIs
While omeprazole is the best-known name in this group, the class includes several widely used alternatives:
- Esomeprazole (Nexium)
- Lansoprazole (Prevacid)
- Pantoprazole (Protonix)
- Rabeprazole (AcipHex)
Doctors often select one of these depending on the severity of symptoms, patient tolerance, and how long treatment is expected to last.
Also Read: Is Donald Trump Showing Early Phonemic Paraphasia? Here’s Why Health Experts Think So
NHS Warning on Long-Term Use
Despite their effectiveness, the NHS has issued a clear warning regarding prolonged, unsupervised use of PPIs. On its official website, the health service cautions:
"Do not take omeprazole for longer than two weeks if you bought it without a prescription. See a GP if your symptoms get worse or do not get better."
The reason for this strict guidance is that long-term or unnecessary use of PPIs may carry hidden risks.
The Risk of Clostridioides difficile Infection
One of the most significant risks linked to extended use of PPIs is a higher chance of developing an infection caused by Clostridioides difficile, often shortened to C. diff.
This bacterium can affect the intestines, producing a range of unpleasant and sometimes dangerous symptoms, including:
- Watery or offensive-smelling diarrhoea (sometimes with blood or mucus)
- Abdominal pain or tenderness
- Fever
- Loss of appetite
- Nausea
In most people, C. diff co-exists harmlessly with other bacteria in the gut. However, when the balance of bacteria is disrupted, particularly after taking antibiotics or acid-reducing drugs like PPIs, it can multiply and release toxins that damage the colon.
The infection is especially risky for older adults, hospital patients, and people with weakened immune systems.
How C. difficile Spreads
The bacteria can also be transmitted through spores that survive on contaminated surfaces, making healthcare settings a common site of infection. This makes it vital to use PPIs under medical supervision, especially for people already vulnerable to infections.
How to Take Omeprazole Safely
Omeprazole is available in tablets, capsules, and liquid form. Some tablets are designed to dissolve in water. The exact dosage and length of treatment depend on the medical condition being treated.
General NHS advice includes:
- Take omeprazole once daily at the same time each morning.
- If prescribed twice daily, take it morning and evening consistently.
- It can be taken with or without food.
Swallow tablets whole with water. Do not crush or chew tablets labelled enteric-coated or gastro-resistant, as this affects how the drug is absorbed.
Also Read: Cough Syrup Row: Death Toll Rise To 22 As 2 More Children Succumb
Patients struggling to swallow pills are advised to speak to a pharmacist, who may suggest alternative formulations.
Duration of Use
While some individuals only need PPIs for a few days, others may require treatment for weeks, months, or even years depending on their condition. This makes it all the more important to balance the benefits against potential risks.
Possible Side Effects
Like all medicines, omeprazole can cause side effects. The most common side effects include:
- Headaches
- Stomach pain
- Nausea or vomiting
- Constipation or diarrhoea
- Excessive flatulence
Although these are typically mild and temporary, the NHS advises patients to stop driving or using machinery if they experience dizziness or vision problems after taking the drug.
Balancing Benefits and Risks
Medical experts stress that while PPIs like omeprazole are highly effective in treating acid-related conditions, patients should not view them as a “forever” medicine without consultation.
For many, these drugs significantly improve quality of life by controlling painful and disruptive symptoms. But the potential risks of long-term use, particularly regarding gut health and infection, underline the need for ongoing medical guidance.
What Patients Should Do
Health authorities recommend that anyone taking omeprazole or other PPIs should:
- Follow the dosage instructions carefully.
- Avoid prolonged self-medication without consulting a doctor.
- Discuss alternative treatments if symptoms persist.
- Seek medical advice promptly if new symptoms appear, especially diarrhoea, fever, or severe abdominal pain.
Erythritol Sweetener Could Be Linked To Stroke Risk, Finds Study

Credits: Canva
Erythritol sweetener, commonly found in most of the food we consume, whether it is a protein bar or energy drink could be linked to stroke risk. While it is considered as a safer alternative to sugar as a natural sweetener, a study from the University of Colorado suggests it could damage cells in the blood-brain barrier.
The blood-brain barrier is brain's security system that keeps the harmful substance off the limits, while letting in nutrients. Research also suggests that it would lead to serious consequences for heart health and stroke risk.
Erythritol Sweetener Risk: What Did The Study Find?
In the latest study, researchers exposed cells that form the blood–brain barrier to erythritol levels typically seen after consuming a soft drink sweetened with the compound. What followed was a cascade of cellular damage that could leave the brain more vulnerable to blood clots, one of the leading causes of stroke.
The researchers found that erythritol triggered intense oxidative stress, overwhelming cells with unstable molecules known as free radicals. At the same time, it weakened the body’s natural antioxidant defences. This double hit impaired normal cell function and, in some cases, led to cell death.
Damage to blood–brain barrier cells is particularly concerning because this barrier plays a crucial role in protecting the brain from harmful substances circulating in the bloodstream. When its integrity is compromised, the risk of neurological injury rises sharply.
Erythritol Sweetener Risk: How It Disrupts Blood Flow Control
Even more troubling was erythritol’s effect on how blood vessels regulate blood flow. Healthy blood vessels constantly adjust their width—expanding when organs need more oxygen and nutrients, and narrowing when demand is lower.
This process depends on a delicate balance between two molecules: nitric oxide, which relaxes blood vessels, and endothelin-1, which causes them to constrict. The study found that erythritol disrupted this balance by reducing nitric oxide production while increasing endothelin-1 levels.
The result is blood vessels that stay constricted longer than they should, potentially restricting blood flow to the brain. This kind of dysfunction is a known warning sign for ischaemic stroke, the most common form of stroke caused by blocked blood vessels.
Erythritol Sweetener Risk: How It Interferes With Body's Clot Defense
The most alarming finding in the study was how body's natural protect against blood clot is disturbed. Under normal circumstances, cells release a substance called tissue plasminogen activator, which is described as a natural 'clot buster', which helps dissolve clots before they become dangerous. However, erythritol could interfere with this protective mechanism and allow clots to persist and cause damage.
Several have shown that people with higher blood levels of erythritol face significantly increased risks of cardiovascular events. In one major study, individuals with the highest erythritol levels were nearly twice as likely to suffer a heart attack or stroke.
However, researchers caution that the experiments were conducted on isolated cells rather than full blood vessels. More advanced models that better replicate human physiology will be needed to confirm the findings.
Erythritol occupies a unique space in the sweetener world. Classified as a sugar alcohol rather than an artificial sweetener, it escaped recent World Health Organization guidance discouraging artificial sweeteners for weight control. Its sugar-like taste has also made it a favorite in “keto-friendly” and sugar-free foods.
FDA Refuses To Review Moderna's Flu Vaccine Application
Credits: Canva
FDA refuses to review Moderna's flu vaccine: The U.S. Food and Drug Administration (FDA) has declined to begin reviewing Moderna’s application for its experimental flu vaccine. The company made the announcement on Tuesday. The decision marks another signal of stricter vaccine oversight under the Trump administration and has already rattled investor confidence, with Moderna’s stock falling nearly 7% in after-hours trading.
Read: CDC Vaccine Schedule: Coverage Falls From 17 to 11 Diseases For Children
Moderna said the FDA’s refusal came as a surprise and contradicted feedback the company had received earlier, before it submitted the application and launched phase three trials for the vaccine, known as mRNA-1010. The company has now requested a meeting with the agency to better understand what it described as an unclear “path forward.”
FDA Refuses: Objections Raised Over Trial Design
According to Moderna, the FDA did not flag any safety or efficacy concerns with the vaccine itself. Instead, the agency objected to the design of the clinical trial—despite having previously signed off on it. Moderna added that the setback would not affect its financial guidance for 2026.
The experimental flu shot had shown encouraging results in phase three trials last year, successfully meeting all primary trial endpoints. At the time, Moderna positioned the stand-alone flu vaccine as a critical step toward developing a combined influenza and COVID-19 vaccine, a key long-term goal for the company.
FDA Refuses: Shifting U.S. Vaccine Policy Landscape
The decision comes amid sweeping changes to U.S. immunisation policy over the past year under Health and Human Services Secretary Robert F. Kennedy Jr., who has long expressed skepticism toward vaccines. Moderna on Tuesday pointed to the FDA’s top vaccine regulator, Vinay Prasad, who returned to the agency in August after being removed earlier.
Prasad currently heads the FDA’s Center for Biologics Evaluation and Research (CBER) and has publicly argued for tighter regulatory standards for vaccines. He has also drawn controversy for comments linking child deaths to COVID-19 vaccines.
FDA Refuses: FDA Letter Cites Comparator Concerns
In a letter dated February 3 and signed by Prasad, the FDA stated that its refusal to review Moderna’s application was solely due to concerns about the trial’s design. Specifically, the agency objected to Moderna’s choice of comparator, arguing that comparing the experimental shot to a standard, approved flu vaccine did not represent the “best available standard of care.”
As a result, the FDA concluded that the study did not qualify as an “adequate and well-controlled” trial under its regulatory definition.
Moderna has strongly disputed this interpretation, arguing that FDA rules do not require companies to use the most advanced or highest-dose vaccine as a comparator in clinical trials.
FDA Refuses: Moderna Pushes Back, Eyes 2026–27 Timeline
In a statement, Moderna CEO Stéphane Bancel said the decision undermines innovation and fails to advance shared public health goals. He emphasized that the trial design had been discussed and agreed upon with CBER before the study began.
Moderna now expects the earliest possible approval for its flu shot to come in late 2026 or 2027, pending regulatory reviews across the U.S., Europe, Canada, and Australia.
The FDA declined to comment, stating it does not discuss regulatory communications with individual companies, reported CNBC.
Measles Cases Cross 14,000 in Mongolia, Mostly Among Partially Vaccinated Children
Credits: Canva
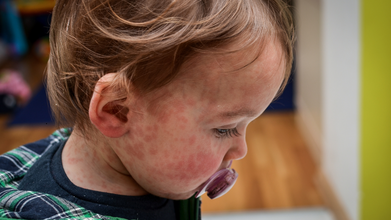
Mongolia is witnessing a sharp rise in measles infections, with the total number of confirmed cases reaching 14,123, according to the country’s National Centre for Communicable Diseases (NCCD). Health officials say the outbreak is largely affecting school-age children, many of whom had received only one dose of the measles vaccine instead of the recommended two.
In a public advisory, the NCCD urged parents to ensure their children complete the full vaccination schedule, warning that partial immunization leaves children vulnerable to a potentially severe and highly contagious disease.
Why Children Are Most Affected
Health authorities noted that the majority of new infections were recorded among children who had not received their second measles shot. While a single dose offers some protection, it is not sufficient to prevent outbreaks, especially in school settings where close contact accelerates transmission.
The NCCD stressed that completing the two-dose regimen significantly strengthens immunity and reduces the risk of community-wide spread.
One of the World’s Most Contagious Viruses
Measles is considered one of the most infectious diseases known to humans. It spreads through direct contact with infected nasal or throat secretions, such as coughing or sneezing, and through airborne transmission in enclosed spaces.
What makes measles particularly dangerous is its ability to remain active in the air or on surfaces for up to two hours after an infected person has left the area. According to health experts, a single measles patient can infect up to 18 other people, making rapid containment extremely challenging once outbreaks begin.
Why Vaccination Still Matters
Vaccination remains the most effective way to prevent measles infection and limit its spread. The measles vaccine is safe, cost-effective and helps the immune system recognize and fight the virus before serious illness develops.
Before the measles vaccine was introduced in 1963 and widely adopted, large-scale outbreaks occurred every two to three years worldwide, causing an estimated 2.6 million deaths annually. Despite medical advances, measles continues to claim lives when vaccination coverage declines.
In 2023 alone, an estimated 107,500 people died from measles, most of them children under the age of five, highlighting the consequences of gaps in immunization programmes.
Recognising the Symptoms Early
Symptoms of measles typically appear 10 to 14 days after exposure. Early signs often resemble a common viral illness and can last up to a week. These include a runny nose, persistent cough, fever, red and watery eyes, and tiny white spots inside the mouth known as Koplik spots.
The most recognizable symptom—a red, blotchy rash—usually appears 7 to 18 days after exposure. It starts on the face and upper neck before spreading downward to the torso, arms, legs, hands and feet over several days. The rash generally lasts five to six days before fading.
A Renewed Call for Prevention
Health officials in Mongolia emphasize that measles outbreaks are preventable. They urge parents, caregivers and schools to prioritize full vaccination and seek medical advice at the first sign of symptoms.
With measles capable of spreading rapidly through communities, authorities warn that completing both vaccine doses is not optional but essential to protecting children and preventing future outbreaks.
© 2024 Bennett, Coleman & Company Limited

