- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
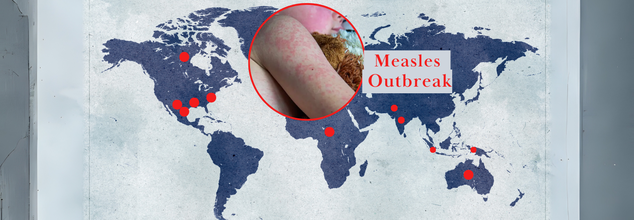
Not Just In US, Measles Outbreak Is Growing Even Across The World- Is It Time To Get A Booster Shot?
Once thought to be eradicated in the US, measles is making a concerning return. With rising cases and recent deaths in the US—the first since 2015—public health officials are on high alert. The Centers for Disease Control and Prevention (CDC) has been tracking the outbreak closely, exacerbated by decreased vaccination rates and increased international travel. The situation is not just in the US; in Australia and Canada, cases are rising, and health authorities across the globe are issuing warnings.
Measles is an extremely contagious and airborne illness that will result in severe complications, such as pneumonia, inflammation of the brain (encephalitis), and even death. The CDC states that nearly 1 of every 5 unvaccinated people who get the virus will need to be hospitalized, and as many as 3 of every 1,000 infected children can die from complications. As vaccination continues to spread, the question remains: Is it time for round two of vaccinations?
The US and global resurgence of measles can be traced to the following factors:
Declining Vaccination Rates
Routine immunization was disrupted during the COVID-19 pandemic, which resulted in declining vaccination rates in children. According to the CDC, the coverage of the MMR (measles, mumps, and rubella) vaccine in kindergarteners declined from 95.2% during 2019–2020 to 92.7% during 2023–2024, making about 280,000 children susceptible to measles.
International Travel
Outbreaks of measles in countries with lower vaccination rates, including some areas of Asia and Europe, risk unvaccinated travelers getting the illness overseas and transporting it back to their native countries. This was reflected in 2019 when imported cases of measles nearly led the US to lose its elimination status.
Misinformation and Hesitancy: Misinformation-driven vaccine hesitancy has been a key driver of decreased immunization coverage. Misconceptions regarding the safety and efficacy of vaccines have dissuaded parents from getting their children vaccinated, raising the threat of outbreaks.
Measles Outbreaks Outside the US
It is not only the US that is experiencing a measles crisis. Australia has witnessed an increase in cases, and health authorities issued warnings in Melbourne and Sydney. Five new cases were recently reported in Victoria, where some were associated with international travel. Residents are being called upon by health officials to watch out for symptoms and seek treatment if they believe they have been exposed to the virus. In New South Wales, a tourist infected with measles traveled to Sydney and attended several venues, and there are fears of community spread.
While measles is no longer endemic in Australia, it still appears as a result of travel-acquired infections. This emphasizes the importance of worldwide vigilance and collaborative public health action to avoid further outbreaks.
Should You Get a Measles Vaccine Booster?
For individuals immunized on the recommended schedule—two doses of the MMR vaccine—there is more than a 95% probability of lifelong immunity against measles. But some adults require a booster dose. People who were vaccinated prior to 1968 might have been given an earlier formulation of the measles vaccine that was derived from an inactivated virus, and not as effective as the current live attenuated vaccine. Adults in this group need to receive at least one dose of the new MMR vaccine. Moreover, the CDC also advises an additional dose of measles vaccine to adults with high-risk exposure, such as college students, healthcare personnel, and those who live with immunocompromised individuals. International travelers must also be fully vaccinated because outbreaks of measles continue to pose a threat in countries with poor immunization coverage. Precautions against these can prevent infection and check the resurgence of measles globally.
Why Is There a Urgent Need for High Vaccination Rates?
Measles is among the most infectious diseases, which can be spread in the air when an infected person coughs or sneezes. For this reason, there needs to be a high percentage of vaccination coverage so that outbreaks are prevented. Public health professionals emphasize that a minimum of 95% of a community should be vaccinated to ensure herd immunity.
In the US, vaccination rates have fallen below this level in recent years, and states such as Texas have had particularly severe outbreaks. This trend is echoed internationally, with several countries having declining immunization rates and following them, resurgence of disease.
What to Do If You've Been Exposed to Measles?
If you think you've been exposed to measles, it's important to move quickly:
- Call your doctor/ a medical practitioner immediately who will be able to check your immunity, give post-exposure prophylaxis if needed, and advise on what to do next.
- Symptoms of measles such as fever, cough, sore throat, and a characteristic rash usually develop 7 to 14 days following exposure.
- Isolation for four days or more after the rash develops is advisable in case you have been diagnosed with measles in order to avoid further transmission.
Measles epidemic- once preventable disease, now poses communities with the risks of declining vaccine coverage and globalization. With cases of measles increasing on several continents, the response is obvious- Now is the time to put immunization first. If you are uncertain about your immunization history, see a health care provider and get another shot—because when it comes to measles, prevention is the best treatment.
Credits: Canva
FDA Greenlights Zevaskyn for Rare Genetic Skin Condition
In a landmark development for patients who live with a rare and painful skin condition, the US Food and Drug Administration or the FDA has approved Zevaskyn (prademagene zamikeracel) for the treatment of recessive dystrophic epidermolysis bullosa or RDEB. This is an inherited disorder that causes the skin to be extremely fragile. I also leads to chronic wounds, bleeding, and tearing even from minor friction or trauma.
As per the 2015 study published in the Journal of Clinical and Aesthetic Dermatology, there are four major subtypes of the skin disorder, which comes from the heterogeneous group of inherited mechanobullous disorder hat is caused by mutation in genes that encode structural proteins in the skin. The overall condition is referred to as epidermolysis bullosa, and one of its type is RDEB, which further comes with two main subtypes of dystrophic EB.
Zevaskyn is now the first and only autologous cell-based gene therapy approved for both adult and pediatric patients living with this life-altering condition.
Why Is This A Breakthrough In Gene Therapy?
Zevaskyn represents a new era in wound care and gene therapy. Unlike traditional treatments that only manage symptoms, this one-time surgical application targets the underlying genetic mutation responsible for RDEB. The therapy uses the patient's own skin cells, which are genetically modified to produce a functional version of the missing COL7A1 gene, critical for anchoring skin layers together.
"Zevaskyn is not just a bandage—it’s a breakthrough that may help change the course of this disease for many," said Madhav Vasanthavada, Ph.D., Chief Commercial Officer at Abeona Therapeutics, the biopharmaceutical company behind the treatment.
How Was It Approved?
The FDA based its approval from the results of two clinical trials: a phase 1/2a study and the pivotal phase 3 VITAL study.
Phase 1/2a Trial: In this study, seven patients with 38 chronic wounds received a single Zevaskyn application. Researchers observed a significant and long lasting improvement at treated sites during the median follow-up of seven years.
Phase 3 VITAL Study: This was a larger study that included 43 patients with large unhealed or non healing wounds. After six months, 81% of those wounds treated by Zevaskyn, showed at least 50% healing, as compared to only 16% in the control group, who had received the standard care.
These outcomes were not only statistically significant but also clinically meaningful, especially for patients who have previously struggled with limited treatment options.
Zevaskyn also showed a favorable safety profile across both studies. No treatment-related serious adverse events were reported. The most common minor side effects were procedural pain and itching, affecting approximately 5% of participants.
"This therapy offers hope for patients and families who have lived too long without effective solutions," said Vasanthavada. “We’re confident in Zevaskyn’s ability to deliver long-term results and are committed to making it widely accessible.”
Access For Patients
To ensure access, Abeona Therapeutics plans to collaborate with both commercial insurers and government payers. The company aims to develop outcome-based agreements that reflect the long-term benefits of a single application of Zevaskyn, reducing the need for repeat procedures or ongoing wound care costs.
With FDA approval, Zevaskyn is set to be a game-changer in the treatment of recessive dystrophic epidermolysis bullosa—offering patients more than just relief, but a meaningful step toward healing.

Credits: Canva
Florida Also Lines Up To Ban Fluoride From Public Water Systems
Florida is on the brink of becoming the second taste to ban fluoride in public drinking water. It will join Utah, which had become the first state to enact the ban just last month. While this was opposed by dentists and national health organizations, who had also warned against this move to lead to more medical problems, dental problems and also affecting low-income communities disproportionately, Spencer Cox, Republican Gov. Signed the legislation regardless.
The Controversial Bill
The bill has received final approval from Florida lawmakers on Tuesday and is now being headed to Republican Governor Ron DeSantis' desk for approval. DeSantis' administration has been an outspoken critic of adding fluoride to community water systems. They have argued that high fluoride levels could potentially affect children's intellectual development. The same concern was quoted by Cox to ban fluoridated water. This is based on the paper published in the medical journal JAMA Pediatrics that concluded that there may be a link between high levels of fluoride and lower Intelligence Quotient (IQ). Their research indicated a possible neurodevelopmental harm to pregnant people or young children if they are exposed to drinking water containing at least 1.5 milligrams of fluoride per litre- a level more than twice what's recommended (0.7 mg/L) for the US water supply. It is important to note that in many American states and Western countries, pregnant women and children receive fluoride from many sources, making their exposure to this mineral way too high from the recommended levels.
However, the study does not the safe levels of fluoridated water which is safe for use. In fact, these levels are also regulated by the US Food and Drug Administration (FDA).
What Do The Republicans Want?
The bill, sponsored by Republican state Representative Kaylee Tuck, does not explicitly mention fluoride but mandates the removal of fluoride and other additives from the state's water systems.
According to Tuck, the legislation focuses on removing additives related to health, rather than water quality itself. "Anything that relates to water quality, removing contaminants, things like that, we're not touching that," she stated. "It's anything that has to do with health, so fluoride, vitamins, whatever else it is."
The Resistance
There has been local resistance to from the local authorities in Florida. The Miami-Date County Mayor Daniella Levine Cava expressed her dismay with this move and stated that this will undermine the overwhelming support of medical professionals for the practice of fluoridating water. She said that ending fluoridation could lead to harmful consequences, especially for the vulnerable families, who would lost access to a cost-effective method of preventing tooth decay.
Fluoridation has been a standard practice in many parts of the U.S. for decades, and it has been credited with significantly reducing the incidence of cavities. According to the Centers for Disease Control and Prevention (CDC), fluoride helps to strengthen tooth enamel and make it more resistant to cavities.
Health Benefits Of Flouride
Fluoride is a naturally occurring mineral that plays an essential role in maintaining strong, healthy teeth. It helps replace minerals lost from tooth enamel due to normal wear and tear. Additionally, fluoride can help reverse early signs of tooth decay by remineralizing the enamel. It also reduces the production of acids by bacteria in the mouth, which further helps prevent plaque buildup.
Beyond dental health, fluoride is also beneficial for bones. It stimulates new bone formation and has been shown to protect against conditions like osteoporosis. As such, fluoride is not only important for dental care but also for overall skeletal health.

(Credit-Canva)
Leukemia Can Now Be Treated By New Immune Cell Treatment
Cancer is an umbrella term for abnormal excess growth that can occur in any part of the body. Leukemia is the cancer of blood, which means there is rapid growth of abnormal blood cells. This growth starts in the bone marrow, which is where your body makes blood. The Cleveland Clinic explains that unlike other cancers, leukemia does not form a mass or tumor that can be detected in a CT scan.
The usual treatment of Leukemia involves Chemotherapy, whether by pill, injection into your vein or a shot under your skin. Another treatment for it is immunotherapy which uses a drug to boost your body’ defense system so that it can fight the cancer itself. Now, a research by American Association for Cancer Research April 2025, has revealed that a pre-made version of immunotherapy can effectively fight blood cancers. This "off-the-shelf" approach offers a potentially faster and easier way to deliver this powerful therapy to patients in need.
Promising Treatment Against Blood Cancer
This new way to treat blood cancer uses special immune cells called natural killer cells, or NK cells. These NK cells have been changed in a lab to have special tools, called CARs, that help them find and kill cancer cells. What's really helpful is that these CAR NK cells can be made ahead of time from healthy people and stored. This means doctors can just take them off the shelf and give them to patients who need them quickly, without having to wait for a treatment to be made just for them, making the whole process much simpler and faster.
The results from using this ready-made CAR NK cell treatment yielded promising results, especially for people with a type of blood cancer called acute myeloid leukemia, or AML. The scientists found that after getting this treatment, some of the patients with AML had their cancer completely disappear. This is called complete remission, and it means there were no signs of cancer left in their blood. These early successes give a lot of hope for a new and better way to treat this difficult disease.
Notably, AML is a very fast-growing and serious cancer. According to American Cancer Society this cancer develops in the myeloid cell, the cells that would normally become white blood cells. This type of cancer develops quickly, hence needs to be treated with the same urgency.
Who Does This Treatment Help?
The first group of patients who received this ready-made CAR NK cell treatment were people whose leukemia had either stopped responding to other treatments or had come back after treatment. These are often the most difficult cases to treat. The fact that some of these patients had such a good response to the SENTI-202 treatment, which is a safety feature to the SENTI-202 cells. It's like a special switch that stops the NK cells from attacking healthy cells in the body. With their cancer completely disappearing, is a very encouraging sign that this new approach could offer hope to patients who have run out of other options.
More Research Needed To Confirm Safety and Effective
The researchers are hopeful about the new treatment and early success for the treatment. They believe that this ready-made approach could lead to new types of immune therapies that are much easier to produce and give to patients. Researchers emphasized that there's a big need for better treatments for AML, and he hopes this new method can become an important option for these patients who often have very limited choices.
It's important to remember that these are just the first results from an ongoing study. The scientists are still enrolling more patients to learn even more about how safe and how well SENTI-202 works. They need to keep studying it to make sure it's a reliable and effective treatment for more people with blood cancers. Before this treatment can be used widely, the findings need to be carefully reviewed and published in a scientific journal.
© 2024 Bennett, Coleman & Company Limited

