- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
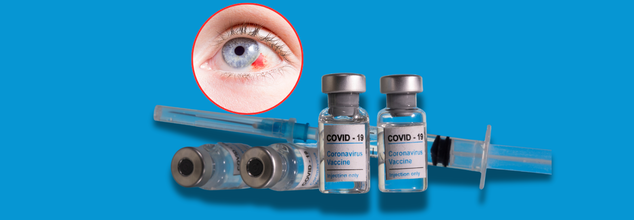
Pfizer COVID-19 Vaccine Linked To Major Eye Dysfunction, Study Finds
Credits: Canva/Reuters
A new peer-reviewed study published recently has sparked alarm regarding the connection between Pfizer's mRNA COVID-19 vaccine and the occurrence of a rare but severe eye disorder, superior limbic keratoconjunctivitis (SLK). In an article published in the Cureus peer-reviewed medical journal, researchers highlighted an emerging trend of eye inflammation and malfunction after COVID-19 vaccination, more so among those with pre-existing autoimmune conditions.
Although the general risk seems to be low, the study emphasizes the significance of post-vaccine surveillance and the need for physicians and ophthalmologists to take recent vaccination status into account when diagnosing unexplained eye symptoms. Pfizer remains silent about the findings of cases.
Also Read: Egg Recall California 2025: How to Protect Yourself Amid Salmonella Cases
Researchers at a Turkish medical institution analyzed 64 individuals—128 eyes—before and after they received both doses of the Pfizer-BioNTech COVID-19 vaccine. Two months after the second dose, the researchers documented measurable changes in the cornea’s endothelium, the innermost layer that plays a critical role in maintaining corneal clarity. Significant changes noted were:
- Thickening of the cornea from a mean of 528 to 542 micrometers
- An 8% reduction in endothelial cells from 2,597 to 2,378 cells/mm²
- A 2% reduction in healthy hexagon-shaped cells
- Greater variation in cell size, which may indicate cellular stress or damage
Although the study had no immediate detection of visual impairment among the participants, the scientists observed these changes as possible harbingers of long-term dysfunction, particularly among individuals with pre-existing eye problems.
Why Is the Corneal Endothelium Important?
The endothelium is a single-cell layer responsible for pumping excess fluid out of the cornea to keep it transparent. Unlike most other cells in the body, these cells don’t regenerate. Once they’re damaged, the surrounding cells stretch to fill the gaps—leading to irregularity in cell shape and size, which was observed in this study. A low cell count or disorganized endothelial layer can result in conditions like:
Corneal edema: Swelling that causes blurred or hazy vision
Bullous keratopathy: Painful blisters on the eye surface
Corneal decompensation: Permanent loss of clarity, potentially requiring a transplant
While healthy adults can often tolerate mild changes, individuals with already low endothelial cell counts, due to aging, Fuchs’ dystrophy, prior surgeries, or eye trauma—could be more vulnerable.
Researchers emphasized that these effects could be temporary, and long-term follow-up is essential to determine whether the changes persist or resolve on their own. They advised individuals with low endothelial counts or past corneal grafts to undergo more frequent checkups following vaccination, including specular microscopy, a non-invasive scan that measures endothelial health.
What We Know About Vaccine's Side Effects
The findings contribute to a broader conversation around vaccine safety, particularly concerning rare but notable adverse effects. In 2021, U.S. regulators added myocarditis and pericarditis warnings to the Pfizer and Moderna vaccines, mostly affecting men aged 16 to 25.
While such side effects remain rare, increased transparency and post-market surveillance have become crucial for maintaining public trust. The Turkish corneal study does not accuse Pfizer of negligence, nor does it dispute the vaccine's life-saving role during the pandemic. However, it adds new dimensions to ongoing risk-benefit assessments, especially for individuals with specific medical histories.
Should You Be Worried?
For most people, especially those without underlying eye conditions, the reported corneal changes likely have little or no practical impact. The measured drop in endothelial cells still kept participants well within the healthy range (above 2,000 cells/mm²).
But if you’ve had previous eye surgery, a corneal transplant, or conditions like Fuchs' dystrophy, it’s worth discussing this study with your ophthalmologist. You may be advised to monitor corneal health pre- and post-vaccination.
Also, while the study used robust diagnostic tools—like Sirius corneal topography and the Tomey EM-4000 specular microscope—the sample size of 64 participants is small. Larger, multicenter studies will be essential to confirm and further interpret the findings.
The researchers made it clear that they are not advising against vaccination. Instead, they are calling for more expansive, longitudinal studies to investigate whether these changes persist, worsen, or reverse over time.
Their key recommendation: monitor individuals who are already at elevated ophthalmic risk. If changes continue to be found months or years after vaccination, guidelines may need adjusting—not to limit vaccination, but to ensure it’s done as safely as possible for all populations.
In the meantime, healthcare providers may consider integrating corneal health screenings into vaccination protocols for high-risk individuals, just as cardiology screenings were emphasized after early reports of vaccine-linked myocarditis.
The Pfizer COVID-19 vaccine remains a vital tool in global health, but emerging research like this adds nuance to the story. The Turkish study’s findings are not a cause for panic, but they are a signal for further investigation, especially in populations with vulnerable eye health.
The COVID-19 pandemic showed how science evolves in real-time—and this study is another chapter in that unfolding story. As always, the best course of action is informed dialogue between patients and healthcare professionals.
If you have a history of eye disease or corneal surgery, consider scheduling an eye exam before and after vaccination—and don’t skip follow-ups. Your vision is worth the extra look.
Melanie Sykes Reveals She’s Living With ‘Post-Traumatic Growth’; Here’s How It Differs From PTSD

Credits: Canva/Melanie Sykes Instagram
Melanie Sykes has shared an encouraging health update, revealing that she is experiencing what she describes as “Post-Traumatic Growth.” On Friday, the former television presenter, 55, spoke openly on Instagram about feeling “vibrating high” after what she called moving through deep trauma. Her comments come amid an ongoing struggle with an autoimmune condition that has caused widespread inflammation and left her almost two-thirds bald.
Melanie Sykes Reveals She’s Living With ‘Post-Traumatic Growth’
In her message, Sykes reflected on how difficult periods do not last forever and introduced the idea of post-traumatic growth to her followers. She explained that it is possible to live with PTSD while also experiencing growth at the same time. “I’m in both camps,” she said, adding that people can hold pain and progress together, as long as they take care of themselves, allow space for grief, process what has happened, and then move forward in ways that bring happiness and meaning.
What Is ‘Post-Traumatic Growth’
Post-Traumatic Growth, often referred to as PTG, describes the positive psychological change that can emerge after someone has faced severe stress or trauma. Rather than simply managing or surviving the experience, people may find new depth in how they see life, feel stronger connections with others, discover fresh possibilities, or undergo spiritual or existential shifts. According to the National Institute of Health, PTG is not about bouncing back to how life was before. Instead, it reflects a deeper transformation that can take a person beyond their pre-trauma sense of self.
How Is Post Traumatic Growth Different From PTSD?
Post-Traumatic Stress Disorder, or PTSD, is marked by ongoing distress following trauma, including intrusive thoughts, avoidance, heightened alertness, and emotional suffering. PTG, on the other hand, refers to the positive psychological changes that can arise after working through trauma, such as greater appreciation for life, stronger relationships, personal strength, and shifts in belief or purpose. As noted by the National Institute of Health, the key difference lies in the outcome. PTSD is considered a mental health disorder, while PTG is a process of meaning-making and growth. Importantly, the two can exist together, with some individuals experiencing distress and growth at the same time.
At its core, PTSD reflects the painful impact of trauma, while post-traumatic growth represents the possibility of positive change that can develop through the long and often difficult path of healing, sometimes alongside or after living with PTSD.
What Condition Is Melanie Sykes Going Through?
Melanie lives with an autoimmune condition, a disorder in which the body’s immune system, meant to protect against infections, wrongly targets its own healthy cells, tissues, and organs. This immune response leads to inflammation and ongoing damage. Common autoimmune diseases include lupus, rheumatoid arthritis, and type 1 diabetes. Although there is no cure, treatment usually focuses on controlling symptoms such as fatigue, pain, and swelling and helping people manage the condition in daily life.
GRAP 4 In Delhi: AQI Crosses 400, Here’s What’s Restricted And What’s Allowed

Credits: Canva
Just hours after GRAP-3 restrictions were implemented, authorities tightened pollution controls in Delhi and surrounding areas on Saturday evening by moving to GRAP-4.
Earlier in the afternoon, the Commission for Air Quality Management (CAQM)—the central agency responsible for monitoring and managing pollution in Delhi and neighbouring states—had imposed GRAP-3 curbs as the Air Quality Index (AQI) surpassed 400, entering the ‘Severe’ category.
By evening, the CAQM reported that the AQI, which was 431 at 4 pm, continued to rise and reached 441 by 6 pm. Under the GRAP system, air quality is divided into four levels: Poor (AQI 201–300), Very Poor (AQI 301–400), Severe (AQI 401–450), and Severe Plus (AQI above 450).
The CAQM stated, “Considering the current air quality trend and to prevent further deterioration in the region, the Sub-Committee on GRAP has decided to implement all measures under Stage IV of the existing GRAP – ‘Severe+’ Air Quality (Delhi AQI > 450) – with immediate effect across the entire NCR. These measures are in addition to the actions already in place under Stages I, II, and III.”
The commission added that the primary reason for the worsening AQI was the weak Western Disturbance moving toward north-west India, rather than local emissions.
GRAP 4 In Delhi: Schools Shift to Hybrid Classes
While schools in Delhi remain open, classes for several grades have moved to a hybrid format, combining both in-person and online learning. The Delhi Directorate of Education (DDE) issued a circular directing all government, aided, and private schools under DOE, NDMC, MCD, and Delhi Cantonment Board to adopt hybrid classes for students up to Class IX and XI wherever online teaching is feasible.
The circular, dated December 13, states: “All Heads of Schools…are directed to conduct classes in schools for children up to Class IX and XI in a ‘Hybrid Mode’ i.e., both physical and online mode (wherever online mode is feasible) with immediate effect until further orders.”
GRAP 4 In Delhi: Are Delhi Schools Open Today?
Yes, all schools under the Delhi Directorate of Education remain open. Students up to Class IX and Class XI can attend classes either physically or online, depending on what they or their parents choose. The hybrid arrangement will continue until further notice. The directive also applies to schools managed by NDMC, MCD, and Delhi Cantonment Board. School authorities have been asked to promptly inform parents and guardians about the arrangements.
GRAP 4 Activated as Pollution Worsens
The move comes after the Commission for Air Quality Management (CAQM) declared Stage 4 of GRAP with immediate effect, as Delhi’s Air Quality Index (AQI) surged into the ‘severe’ category. At 6 am on Sunday, the AQI in the city stood at 462, according to the Central Pollution Control Board’s Sameer app.
GRAP 4 In Delhi: Restrictions Under Stage 4
Stage 4 maintains all measures from Stage 3 and introduces stricter curbs to limit pollution. Among the new restrictions is a total ban on truck traffic entering Delhi, except for vehicles carrying essential goods or providing critical services. Trucks powered by LNG, CNG, electricity, and BS-VI diesel are exempt from the ban.
As UK Faces A ‘Tidal Wave’ Of Flu, Here’s Who Should Get The Flu Vaccine

Credits: Canva
At-risk groups are being encouraged to get their flu jabs as concern grows over rising flu cases across the UK. The NHS has warned of a possible “tidal wave” of infections after flu activity began earlier than usual this season, with some schools already forced to shut because of outbreaks.
A post shared this week by the UK Health Security Agency (UKHSA) on X urged people to take vaccination seriously. It said: “Help protect yourself with a flu vaccine, it’s just like protective armour for those with long-term health conditions like #kidney disease. Stay strong. Get vaccinated.”
What Is The H3N2 Flu Strain?
Health officials have described the newly emerging H3N2 flu strain as particularly “unpleasant” and warned that the NHS may be heading toward a heavy surge in cases in the run-up to Christmas.
Flu-related hospital admissions have already risen by 56 per cent compared with the same period last year, and specialists say the worst of the season may still lie ahead. In response, health leaders have advised people to wear a face covering in public if they are feeling unwell and have urged everyone who qualifies for a flu jab to get vaccinated as soon as possible.
How Can Flu Vaccine Protect From the Flu?
Flu vaccines are meant to protect against influenza, which can be dangerous and even fatal for some groups. Every autumn or early winter, the NHS rolls out the flu jab programme for people who are more likely to develop serious complications from the virus.
Eligibility For Flu Vaccine In UK
According to official UKHSA guidance, six main groups became eligible for the flu vaccine from September 1 this year, while another six groups were added from October 1.
From September 1, this included:
- Pregnant women
- All children aged two or three years old on August 31, 2025
- Children with certain long-term health conditions (aged six months to under 18 years)
- Primary school-aged children (from reception to Year 6)
- Secondary school-aged children (from Year 7 to Year 11)
- All children in clinical risk groups aged from six months to under 18 years
And from October 1, 2025, this included:
- Everyone aged 65 years old and over
- Anyone aged 18 to 65 with long-term health conditions
Health and social care workers may also be offered the flu jab through their workplace. The NHS says it is generally safe to have the flu vaccine at the same time as other jabs, including COVID-19 and shingles vaccines.
The RSV vaccine is usually given separately, but NHS guidance notes that a doctor may decide to give both vaccines together in certain situations.
Super Flu Cases In UK
Amid the recent rise in flu cases, a Downing Street spokesperson also reiterated guidance this week, saying: “There is long-standing guidance in place for people on a range of measures they can consider taking to help limit the spread of winter bugs if they have flu-like symptoms.
“This is neither new nor an instruction but simply something people can consider when trying to limit the spread of winter respiratory illnesses.”
Citing a previous Mirror report, they added: “It’s been a long-standing position. The best defence against flu is the vaccine, which is why we’re ramping up our vaccination efforts this winter with almost 17 million flu jabs already delivered, which is 350,000 more than this time last year.”
The NHS App can be used to check whether you are eligible for a flu jab. Vaccinations are available through GP surgeries, selected pharmacies, maternity clinics, and care homes.
© 2024 Bennett, Coleman & Company Limited

