Scientists Behind Life-Changing Cystic Fibrosis Treatment That Extends Life By Decades Win Medical ‘Nobel’ Prize
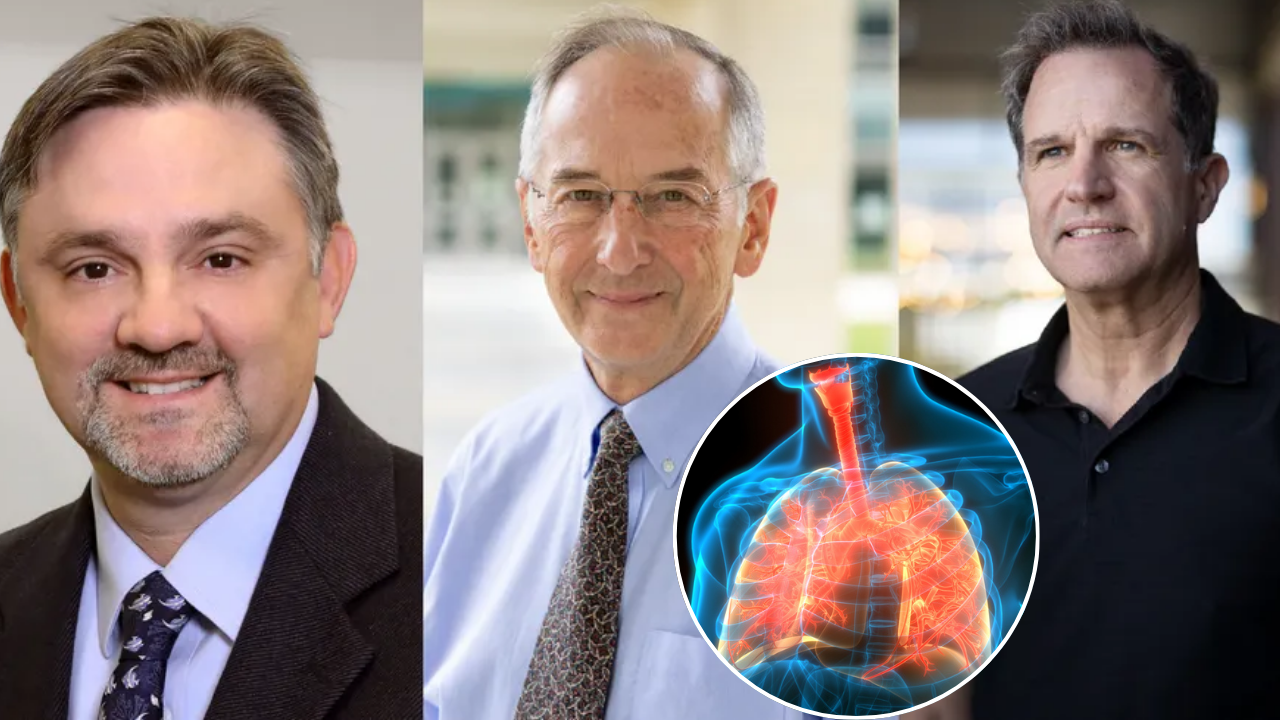
SummaryThe 2025 Lasker Award, often called the “American Nobel,” was awarded to scientists who developed a groundbreaking cystic fibrosis treatment. Their therapy, now a standard of care, extends patients’ lives by decades, marking a historic medical achievement.
End of Article
