- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Scientists Derive Unique Method To Counter Malaria—Feed Mosquitos With Drugs

Credit: Canva
What if, instead of killing mosquitoes to stop malaria, we simply cured them? Harvard scientists have come up with a unique solution—giving mosquitoes anti-malarial drugs could turn these notorious disease-carriers into harmless biters. Malaria—a parasitic disease spread by female mosquitoes—causes nearly 600,000 deaths annually, most of them in children. Traditional prevention efforts have focused on insecticide-coated bed nets, which work by creating a physical barrier and killing mosquitoes that land on them. Many more solutions have emerged in modern times, but the latest proposal asks for curing and not killing the mosquitoes.
What Is Malaria?
Malaria is a life-threatening disease caused by Plasmodium parasites that are transmitted through the bites of infected Anopheles mosquitoes. Common symptoms include high fever, chills, headache, nausea, vomiting, muscle pain, and fatigue. In some cases, especially when untreated, malaria can cause severe complications such as organ failure, difficulty breathing, or even death. The symptoms typically appear 10 to 15 days after being bitten and can resemble those of the flu, making early diagnosis and treatment crucial.
Scientists Identify Two Drugs That Kill Malaria Parasites
To address the lowering resistance to drugs, Harvard researchers tested a variety of drugs on malaria-infected mosquitoes and identified two that kill all parasites when absorbed through the insect's legs. The idea is to add these drugs to bed nets, so even if a mosquito survives contact, it will no longer be able to spread malaria. Study co-author Alexandra Probst calls the research, published in the journal Nature, a novel approach, noting that the malaria parasite is less likely to develop resistance to these drugs due to the limited number found in each mosquito compared to an infected human.
Lab results look promising: The drug treatment lasts up to a year on treated materials, potentially making it a durable and cost-effective alternative to current methods. The next phase—testing these drug-coated nets in real-world conditions—will begin in Ethiopia. Results aren't expected for at least six years, but the hope is to eventually combine both drugs and insecticides on nets, providing a two-pronged strategy against malaria transmission. "Malaria control desperately needs innovation," says study co-author Flaminia Catteruccia in a release. "This is a momentous step forward in the development of a new mosquito-targeted malaria control strategy."
Here's How You Can Identify Malaria
Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries and is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening.
According to the World Health Organisation (WHO), mild symptoms include fever, chills and headache. Severe symptoms include fatigue, confusion, seizures, and difficulty breathing. Infants, children under 5 years, pregnant women and girls, travellers and people with HIV or AIDS are at higher risk of severe infection.
FDA Approves First Neuroimmune Modulation Device for Rheumatoid Arthritis

Credits: Setpoint Medical
The U.S. Food and Drug Administration (FDA) has granted approval to the SetPoint System, a neuroimmune modulation device designed to treat adults with moderate-to-severe rheumatoid arthritis (RA). This marks a new, device-based approach for patients who have limited options due to inadequate response or intolerance to current advanced therapies.
Who Can Benefit?
The SetPoint System is intended for people whose RA is not effectively controlled with, or who cannot tolerate, advanced treatments such as biological and targeted synthetic disease-modifying antirheumatic drugs (DMARDs).
What Is Rheumatoid Arthritis (RA)?
RA is an autoimmune disease that causes painful joint inflammation, stiffness, and long-term joint damage if left untreated. While existing medications can help, some patients experience insufficient relief or serious side effects.
How the Device Works
The SetPoint System is an implantable neurostimulation device that delivers a brief, daily dose of electrical stimulation to the vagus nerve. This nerve plays a key role in regulating inflammation. By activating the body’s innate anti-inflammatory and immune-restorative pathways, the therapy aims to reduce disease activity without the systemic side effects associated with some drug treatments.
Once implanted, the device is programmed to deliver therapy automatically on a set schedule. It can remain in place and function for up to 10 years, offering a long-term treatment option without the need for daily medication intake.
Evidence from the RESET-RA Study
The FDA’s approval is based on results from the RESET-RA trial, which involved 242 patients. Participants were randomly assigned to receive either the SetPoint System or a sham (inactive) device.
The trial met its main goal, showing that more patients using the SetPoint System achieved at least a 20 percent improvement in symptoms, known as the ACR20 response, at three months, compared to those in the control group. Benefits in response rates and disease activity were sustained through 12 months of follow-up.
Remarkably, by the 12-month mark, three out of four participants had not used biologic or targeted synthetic DMARDs.
Safety Profile
The device placement procedure is minimally invasive and performed on an outpatient basis. The therapy was generally well tolerated, with a low rate of related serious side effects, reported in only 1.7 percent of patients in the trial.
Expert POV
Dr. Mark Richardson, principal investigator of the RESET-RA study and professor at Harvard University, said the approval reflects a promising new direction for autoimmune disease treatment.
“The approval of the SetPoint System highlights the potential of neuroimmune modulation as a novel approach for autoimmune disease, by harnessing the body's neural pathways to combat inflammation,” Dr. Richardson said in a statement. “After implantation, the SetPoint device automatically administers therapy daily, simplifying care for people living with RA.”
With this approval, the SetPoint System becomes the first FDA-approved neuroimmune modulation device for RA, adding a non-drug option to the treatment landscape. For patients struggling with medication side effects or insufficient relief, it offers a potentially life-changing alternative that works with the body’s own systems to restore immune balance.
Eli Lilly’s New Weight Loss Pill Helped People With Obesity Shed Nearly 15% In Late-Stage Trials

Credits: Canva
Eli Lilly’s experimental weight loss pill, orforglipron, may soon shift the landscape of obesity treatment. In a Phase 3 clinical trial that spanned more than 3,000 adults, the once-daily pill helped participants lose an average of 12.4% of their body weight over 72 weeks—with minimal lifestyle restrictions and no needles involved.
The company says these findings mark a turning point in the battle against obesity, one of the most pressing global health issues today. Here’s how this oral medication is poised to change everything we know about weight loss treatment.
According to Eli Lilly’s preliminary data, adults who took the highest dose of orforglipron lost an average of 27.3 pounds (12.4% of their body weight) over roughly a year and a half. Nearly 60% of those participants lost at least 10% of their weight, and almost 40% lost at least 15%.
These results are on par with injectable GLP-1 medications like Zepbound and Mounjaro—both also made by Eli Lilly—which have become blockbuster drugs in the obesity and diabetes market.
Dr. Céline Gounder, editor-at-large for public health at KFF Health News, put it bluntly in a CBS interview: “This is what we see with injectables. It’s impressive that a pill could match that.”
The key distinction? Orforglipron is not a peptide-based medication. That makes it easier for the body to absorb and eliminates the need for the strict dietary rules that apply to oral peptide drugs like Novo Nordisk’s Rybelsus.
How GLP-1 in Pill Form Works?
Orforglipron belongs to a class of medications called GLP-1 receptor agonists. These drugs mimic a gut hormone that plays a critical role in appetite regulation and glucose metabolism. They slow down stomach emptying, curb hunger signals in the brain, and help stabilize blood sugar levels.
Until recently, the most effective GLP-1 medications had to be injected, often weekly. Eli Lilly’s innovation—compressing this powerful mechanism into an oral pill—is a major step forward.
Unlike Rybelsus, the only approved GLP-1 pill on the market (which requires fasting and water restrictions), orforglipron is designed to be taken at any time of day, with or without food or water. That alone could make a big difference for patient compliance and everyday ease of use.
Are There Significant Side Effects For This Pill?
The benefits are clear, but what about the risks? As with other GLP-1-based drugs, gastrointestinal side effects were common. The most reported issues included nausea, diarrhea, constipation, and vomiting. Between 29% and 34% of participants experienced nausea—slightly higher than the 25–29% range seen with injectable Zepbound.
More significantly, about 10% of those on the highest dose had to drop out of the study due to side effects. While not uncommon in drug trials, it’s a notable figure for clinicians to weigh when considering patient suitability.
Still, experts say the side effect profile remains consistent with what is expected from GLP-1 medications. Importantly, orforglipron was also associated with improved markers of cardiovascular health, such as lower cholesterol and blood pressure.
What Makes Orforglipron Stand Out?
One of the most promising aspects of this pill is its potential to support long-term weight maintenance. Eli Lilly is also studying orforglipron’s role in helping people who initially lose weight on injectable drugs but are looking for a more manageable, sustainable option moving forward.
In essence, this pill could serve as a bridge—or even an off-ramp—for those who can’t or don’t want to stay on injectable medications forever. The convenience factor is huge.
There’s also the question of cost. Manufacturing pills is typically cheaper than producing injectables, and while pricing hasn’t been announced yet, orforglipron could offer a more affordable option for millions of people who need access to effective obesity care.
Eli Lilly has announced plans to submit orforglipron for regulatory approval by the end of 2025, with hopes of a global launch to follow soon after. If approved by the FDA and other agencies, the drug could hit the market in 2026.
This timeline is aggressive but realistic, given the urgency of the obesity crisis. More than 40% of American adults are classified as obese, according to CDC data. The economic burden and health consequences—including increased risk of heart disease, stroke, diabetes, and certain cancers—are staggering.
The public health need is urgent, and the demand for safe, convenient, and effective weight-loss treatments is at an all-time high. A pill like orforglipron, if approved, could help close that gap.
Despite the promising clinical data, not everyone is convinced just yet. Eli Lilly’s stock fell nearly 14% on the day of the announcement. Some investors expected even greater weight-loss figures or a better side effect profile.
But experts caution against reading too much into the stock market’s knee-jerk reactions. The potential of a widely accessible, non-peptide, once-daily GLP-1 pill is enormous—and it could spark a new era in weight management.
If successful, orforglipron will likely intensify the competition with Novo Nordisk, whose Wegovy and Ozempic have dominated the obesity and diabetes space for the past few years. The race is now on for the first truly scalable, cost-effective oral GLP-1 option—and Lilly may have just edged ahead.
Eli Lilly’s orforglipron isn’t just another weight-loss drug. It’s a potential paradigm shift in how we approach chronic weight management.
If it clears the final regulatory hurdles and delivers on its early promise, orforglipron could bring highly effective, user-friendly weight-loss treatment to millions of people—no needles, no meal timing, no fuss. That’s the kind of change the obesity crisis has been waiting for.
Could Lithium Deficiency In The Brain Trigger Alzheimer’s?
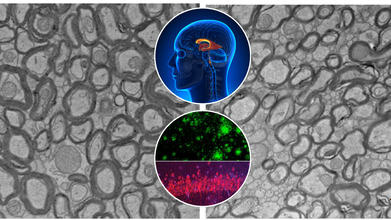
Credits: Yankner Lab/Harvard Medical School
Alzheimer's disease has confounded scientists and destroyed millions of families globally. While advances have been made toward grasping the disease's biological markers—amyloid plaques, tau tangles, and neuroinflammation therapies have generally fallen far short of significant reversal or prevention. A recent study at Harvard Medical School now indicates something remarkable: a natural trace element lithium could be an overlooked piece to the Alzheimer's puzzle.
The study, which appeared in Nature, identifies that lithium is not only a psychiatric medication. It occurs naturally in the human brain and could be necessary for shielding brain cells from aging and degeneration. In mice, the lack of lithium hastened the development and severity of Alzheimer's-like symptoms. More importantly, restoring lithium—specifically in a form that avoids getting trapped by amyloid beta reversed brain damage and brought back lost memory.
What this really means is that lithium isn’t just a potential treatment. It could help explain why Alzheimer’s develops in the first place.
Alzheimer’s doesn’t affect everyone equally. Some people accumulate classic markers like amyloid plaques or tau tangles in their brains but never develop dementia. Others experience sharp cognitive decline despite only mild physical changes.
Dr. Bruce Yankner and his team at Harvard’s Blavatnik Institute believe that lithium levels may be part of the answer. Their ten-year study shows that natural lithium in the brain—like other vital nutrients such as iron or vitamin C—plays a central role in protecting neurons. When levels drop, the shield weakens, leaving the brain vulnerable to damage.
Using advanced spectroscopy on postmortem brain tissue from thousands of individuals, the researchers found that lithium levels were high in cognitively healthy people but markedly reduced in those with mild cognitive impairment or Alzheimer’s. These changes appeared before significant brain damage had occurred, suggesting lithium depletion might kickstart the disease—not just accompany it.
Lithium is well-known in psychiatric medicine. It's a gold-standard treatment for bipolar disorder and is also used in some forms of depression and schizophrenia. At high doses, it stabilizes mood by modulating brain chemicals like serotonin. But its therapeutic window is narrow: too much can lead to kidney or thyroid issues, especially in older adults.
What’s different here is the discovery that tiny, natural levels of lithium—one-thousandth the dose used in psychiatry—may be enough to maintain healthy brain function and prevent neurodegeneration.
“It’s the first time anyone’s shown that lithium exists at a biologically meaningful level in the brain, without administering it as a drug,” Yankner explains. “That’s a game-changer.”
The study used Alzheimer’s-prone mice to examine how lithium levels influence disease progression. When fed lithium-deficient diets, these mice developed early-onset symptoms brain inflammation, memory loss, and accelerated aging. Their brains showed more amyloid plaques, impaired microglial activity, and damage to neuron-protecting myelin.
Conversely, when researchers gave the mice a novel compound called lithium orotate—a version of lithium that bypasses the traps laid by amyloid beta—the mice not only stabilized, they reversed their cognitive decline. In some cases, older mice regained previously lost memories.
The compound worked at ultra-low doses and caused no observable toxicity, even when administered over the animals’ entire lifespans.
Why Lithium Gets Trapped in the Brain?
One of the most surprising discoveries was that amyloid beta binds to lithium, sequestering it and preventing it from doing its job. This happens early in the disease process, even before symptoms arise.
By identifying lithium compounds that evade this trap, researchers opened the door to a new treatment path. Lithium orotate appears to preserve lithium's benefits without falling victim to amyloid beta interference—something that traditional compounds like lithium carbonate fail to do.
This finding could explain why previous clinical trials using conventional lithium compounds in Alzheimer’s have had mixed results or were abandoned due to side effects.
Can Lithium Prevent Alzheimer’s?
This new perspective brings up a big question- could lithium be used not just to treat, but prevent Alzheimer’s? Yankner and his colleagues believe so.
Their findings align with earlier population studies showing lower dementia rates in areas with naturally higher lithium levels in drinking water. The new study, however, goes further. It not only confirms that lithium is present in the brain naturally, but also shows what happens when it's lost—and how replenishing it can make a difference.
This opens the door to new screening methods. If lithium levels can be measured through a simple blood test, they could become a biomarker for early Alzheimer’s risk, much like cholesterol for heart disease.
What Is Lithium Used For?
Lithium is a naturally occurring trace mineral found in soil, water, and certain foods. While it's most widely known as a psychiatric medication—used in higher pharmaceutical doses to treat bipolar disorder and stabilize mood—its nutritional role is lesser known. In micro amounts, lithium appears to influence brain health, cognitive function, and emotional regulation. Some researchers suggest it may have neuroprotective properties, helping to shield the brain from age-related decline, though more data is needed.
What Happens When You Lithium Deficiency?
Although there’s no officially recognized “lithium deficiency” like there is for iron or calcium, emerging research hints that extremely low levels of lithium intake may be associated with higher rates of mood disorders, neurodegenerative conditions, and even increased mortality. Some ecological studies have shown that populations consuming higher natural levels of lithium in drinking water report lower suicide rates and better mental health outcomes. This doesn't mean everyone should start supplementing with lithium, but it does raise important questions about its role in overall neurological and psychological well-being.
Are Human Trials Next?
While the findings in mice are compelling, the leap to human treatment requires rigorous clinical trials. Lithium orotate has not yet been tested in Alzheimer’s patients, and its long-term safety in this context remains unproven.
Still, the research offers a rare blend of explanatory power and therapeutic promise—something that’s often lacking in Alzheimer’s science.
“If this pans out in human trials, it could fundamentally shift how we detect, prevent, and treat Alzheimer’s,” Yankner says. “It’s the most far-reaching effect I’ve ever seen from a single compound.” But he also urges caution. People should not self-medicate with lithium. Over-the-counter lithium supplements are unregulated and could be dangerous, especially at the wrong dose or in older adults with kidney conditions.
This isn’t just another Alzheimer’s study focused on a single protein or pathway. It proposes a unifying mechanism—a missing nutrient that may drive the disease and also offer a way back.
Lithium, once viewed solely as a psychiatric drug, may turn out to be something much more profound: a fundamental player in brain health, a natural buffer against degeneration, and perhaps a key to one of the most confounding diseases of our time.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Do not begin lithium supplementation without speaking to a qualified healthcare provider.
© 2024 Bennett, Coleman & Company Limited

