- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Top Vaccine Scientist Dr. Peter Marks Ousted From FDA- Will This Shake-Up Put Vaccine Safety At Risk?

Image Credit: Canva
Dr. Peter Marks, one of the top FDA's leaders in vaccine regulation, is stepping down from his position as director of the Center for Biologics Evaluation and Research (CBER). His departure has sparked a debate about the future of vaccine regulation and public health policy. Marks was instrumental in leading vaccine approvals, including during the pandemic brought on by COVID-19. As the FDA makes decisions along this transition, experts wait and monitor how it could potentially affect vaccine safety and public trust.
As the head of the Food and Drug Administration's (FDA) Center for Biologics Evaluation and Research (CBER), Marks Marks played a crucial role in ensuring vaccine safety and efficacy, especially during the COVID-19 pandemic. With his announced departure, concerns are mounting over about the future of vaccine regulation in the U.S. and its potential to affect global public health.
Dr. Peter Marks' Resignation or A Forced Exit?
Dr. Marks' April 5 resignation was not voluntary, according to several sources. He was reportedly told: resign or get fired. His exit occurs under the tenure of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr., a highly publicized vaccine critic.
In his resignation letter, Marks expressed deep concern about the direction of vaccine oversight under Kennedy, writing that undermining confidence in vaccines is "irresponsible, detrimental to public health, and a clear danger to our nation’s health, safety, and security." His letter further suggested that scientific integrity was at risk, accusing Kennedy of prioritizing misinformation over truth and transparency.
Since 2016, Marks has served as the head of the FDA's CBER division, guiding the approval of life-saving vaccines, such as the expedited development and authorization of the first COVID-19 vaccines under Operation Warp Speed. His work introduced mRNA technology into vaccines, which have transformed vaccine development and are being investigated for use against a variety of diseases other than COVID-19, including influenza and cancer.
Under his direction, the FDA also approved the first self-administered flu vaccine, offering a new level of protection against flu season. His time at the agency has been characterized by a focus on scientific integrity and transparency, which experts now worry will be undermined.
Kennedy's Anti-Vaccine Influence
Kennedy has been among the loudest anti-vaccine voices, often issuing inflammatory and widely discredited statements. He has challenged the safety of the COVID-19 vaccine and has been a leading force in attempts to undermine public confidence in vaccination campaigns. In 2021, he submitted a citizens petition requesting that the FDA withdraw COVID-19 vaccine authorizations, deeming them "the deadliest vaccines ever made."
His distrust of COVID-19 vaccines is not an isolated incident. Kennedy has also questioned the measles-mumps-rubella (MMR) vaccine, despite the U.S. experiencing the biggest measles outbreak since 2019. During a recent interview, he asserted that the MMR vaccine "does cause deaths every year," something refuted by the Infectious Disease Society of America, which has identified no deaths attributed to the vaccine in healthy people.
Will Vaccine Safety Be Compromised?
Public health officials are sounding the alarm that Marks' resignation could result in a radical change in vaccine regulation and policy. The FDA's CBER branch regulates not only vaccines but also blood products, gene therapies, and allergenic products. Any loss of its regulatory authority could have sweeping implications.
Issues regarding Kennedy's leadership will result in studies that will misleadingly associate vaccines with autism, a theory consistently disproven by scientific studies. Professionals caution that future research may misleadingly imply a connection between vaccines and autism, which could heighten public fear, reduce vaccination rates, and trigger further disease outbreaks.
Also, Dr. Ashish Jha, dean of the Brown University School of Public Health, characterized Marks' ouster as a loss for the FDA. "To push him out makes the FDA immensely weaker, less effective. This is not how we make America healthy," he posted on X (formerly Twitter).
The Risk of Public Health Setbacks
Marks' resignation can't have happened at a more important time. The U.S. is reeling from the increasing rate of vaccine-preventable diseases. The current multistate outbreak of measles, with its ferocity in Texas, highlights the severity of sliding vaccination rates. According to a report by the World Health Organization (WHO), more than 100,000 unvaccinated children have died last year in Africa and Asia as a result of complications related to measles. These numbers are a hard reminder of how devastating things become when misinformation surrounding vaccines spreads.
At the same time, HHS has allegedly requested the Centers for Disease Control and Prevention (CDC) to reexamine the vaccine-autism connection, in the face of overwhelming scientific agreement that no such connection exists. The action further stoked concern that Kennedy's power might result in policy that negates a half-century of scientific advancement.
What's Next for the FDA and Public Health?
With Marks' departure, the FDA will have a vacuum of leadership at a moment when public health institutions' trust is already weakened. His replacement will have to contend with upholding scientific integrity while dealing with pressures from an administration that seems more and more politicized to counter anti-vaccine sentiment.
The next few months will tell if the FDA can resist political pressure and remain committed to its mission of safeguarding public health. For the moment, experts are calling for caution, stressing the need to protect the scientific standards that have long shaped vaccine regulation in the U.S.
Marks' exit is not simply a house-cleaning in bureaucratic circles—more importantly, it signals a possible turning point in how vaccine science is weighed, approved, and disseminated to the general public. Will this be a catalyst for public health disaster or not, the struggle for vaccine science is just beginning.
‘It Ends With Us’ Author Colleen Hoover Reveals Cancer Diagnosis As She Undergoes Radiation

Credit: Instagram/ColleenHoover
Colleen Hoover, the best-selling author of 'Regretting You' and 'Verity', has revealed that she has undergone treatment for cancer.
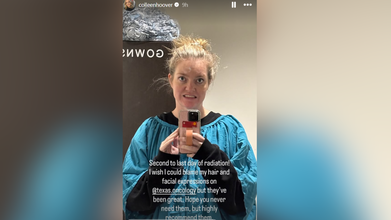
On January 12, Hoover announced via her Instagram Stories that she has one more day of radiation left at Texas Oncology.
"Second to last day of radiation," she captioned the post on her Instagram Stories. "I wish I could blame my hair and facial expressions on @Texas.Oncology, but they've been great. Hope you never need them, but highly recommend them."
In December 2025, the 46-year-old writer wrote on her Facebook page that she had been diagnosed with cancer and had undergone surgery. The film producer also noted that she would only need radiation, but not chemotherapy as a treatment plan.
Hoover said she had been in Canada filming Reminds of Him when she noticed she “had recurring health issues that I continued to put off until the movie was finished" and a check-up revealed that she had developed cancer.
What Kind Of Cancer Does She Have?
While she is yet to reveal which kind of cancer she is receiving treatment for, Hoover has confirmed it was not caused by family genes, HPV or excessive hormones.
In a Facebook post from January 9, she noted that the reason for her illness is 'more than likely' to be environmental/lifestyle, which can be credited to a lack of exercise, poor diet and stress.
"I’m happy and grateful to be alive but I hate vegetables. I hate when I have to get off the couch. I hate sweating. I hate when science is right. If you see me at the gym, don’t even tell me good job. If you see me at a restaurant eating grilled chicken and drinking water, I’m probably real mad about it," she wrote in a heartfelt moment.
Why Has Hoover Been Missing From Public Eye?
Hoover's health updates and cancer treatment come amid a series of cancelled public events. In October 2025, she announced she would not attend the premiere of Regretting You, her latest movie adaptation.
In a sentimental Instagram post, she told her fans: "I’m super bummed, but am having an unavoidable surgery and can’t travel for a while,” Hoover wrote in her Instagram post at the time. I’ll live vicariously through you guys. So sad to miss this movie release and premiere, but so grateful to all the actors and the team who put this together.”
Woman Down is Hoover’s next book release set for January 13. However, the author has had to tell fans that a book-signing tour has not been set up yet and she will not be meeting with the public for now.
“I wanted to make this post and be transparent about why that is. I’m not saying I won’t be up to doing at least one signing, but I just won’t know until I know," she said.
Meanwhile, throughout 2025, production on her hit novel, Verity, has begun in 2025, with Anne Hathaway and Josh Hartnett, being photographed during scenes last February. Additionally, her third movie adaptation, Reminders of Him, is also set to hit theaters on March 13, 2026.
It remains unknown if she will be attending the premiere for Reminders of Him.
Mattel Launches First-Ever Barbie With Autism

Credits: Mattel
Barbie. A name too familiar for everyone growing up, now Mattel Studio has launched its first autistic Barbie. This is after Greta Gerwig's 2023 blockbuster movie that introduced the concept of diverse characters of Barbie, that Mattel launched its first Barbie with type 1 diabetes. The studio is definite about creating a diverse range of Barbie character, as an animated Barbie film too is in the development.
Why Is The Launch Of Autistic Barbie Important?
Autistic Barbie is the latest addition to Barbie's Fashionistas range, which is designed so more children can "see themselves in Barbie". The Barbie is created in collaboration with the US charity the Autistic Self Advocacy Network. The Barbie is designed to represent the ways autistic children may have experienced, including the way they communicate.
Barbie With Autism: What Are Her Features?

The Barbie's eyes gaze slightly to one side, which could represent how some autistic people avoid direct eye contact. The Barbie also comes with completely bendable elbows and wrists, which enables her repetitive physical movements such as stimming and hand-flapping that help people with autism to process sensory information, or even express excitement.
She also holds a pink fidget spinner on her finger, which helps her reduce her stress, wears noise-cancelling headphones, also in pink, to reduce sensory overload, and carries a pink tablet with symbol-based augmentative and alternative communication buttons on its screen that helps with her everyday communication.
Barbies earlier came in one-size-fit-all, often lacking the diversity and representation, this Barbie, however, wears a loose-fitting purple pinstripe A-line dress. This has minimized fabric to skin contact, and her shoes have flat soles that promotes stability and ease of movements.
Read: Hundreds Of US Children Have Type 1 Diabetes, Now Their Barbie Has It Too
Barbie's Representation Matters
The first Barbie came in 1959, and until 2019, there were no dolls with disabilities. Now, there are Barbies with diabetes, blind dolls, and Barbies with wheelchairs, Down syndrome, prosthetic limb, vitiligo, and hearing aids. There is also a Ken doll with a prosthetic leg, and another one who uses a wheelchair with a ramp, and one with hearing aids.
Jamie Cygielman, the global head of dolls at Mattel while announcing the autistic Barbie said, "Barbie has always strived to reflect the world kids see and the possibilities they imagine, and we’re proud to introduce our first autistic Barbie as part of that ongoing work. The doll helps to expand what inclusion looks like in the toy aisle and beyond because every child deserves to see themselves in Barbie."
She also added: “We engaged with the autistic community throughout the design process, always mindful that autism is experienced differently by every individual and is not always visible. The elements of this doll reflect how some people on the spectrum may experience and express the world, and we hope that by partnering with influential voices within the community, Barbie can continue to showcase a broader range of authentic experiences.”
What Is Autism?
As per the American Psychiatric Association, ASD is a complex developmental condition involving persistent challenges with social communication, restricted interests and repetitive behavior. While autism is considered a lifelong condition, the need for services and supports because of these challenges varies among individuals with autism.
As per the Centers for Disease Control and Prevention, an estimated one in 36 children have been identified with ASD.
Philippines' Northern Mindanao Sees An Increase In Measles Infections
Credits: Canva
The Department of Health (DOH) reported 411 measles infections in Philippines' Northern Mindanao in 2025, which was a 11% increase from 371 cases in 2024. Health officials are now warning people that the situation may get worse in 2026, as rate of unvaccinated children in the region also rise.
In 2025, 75% of the patients were unvaccinated.
In order to prevent outbreaks, DOH-Northern Mindanao and local health offices said that they will be deploying teams to administer Measles-Rubella (MR) vaccines to children and people aged six to 59.
“There are (measles) outbreaks in various parts of the country because of low (immunization) coverage and that includes our region,” said Germaine Labadan, head of the DOH-X Family Health Cluster.
In 2025, only 56.7% of Northern Mindanao's eligible population received MR shots, which led to a herd immunity well below the target of 95%.
Cagayan de Oro, a highly urbanized city, recorded the region’s highest measles-rubella (MR) coverage at 87.21%, while Bukidnon lagged at 49.71%. Coverage in other areas remained low, with Misamis Oriental at 51.87%, Misamis Occidental at 52.05%, Iligan at 52.55%, and Camiguin at 53.73%.
As part of this year’s Measles-Rubella Supplemental Immunization Activity (MR-SIA), children will be given booster MR vaccines along with Vitamin A supplements. The 21-day campaign will run from January 19 to February 13.
“This is open to all eligible, regardless of the immunization status of the child because the vaccine serves as a booster,” Labadan said. This also includes children who completed the first and second doses. “Parents may present the immunization booklet, but it is not necessary because all will be given a new one,” she said.
What Is Measles?
Measles, also known as rubeola, is a highly contagious viral illness that typically causes fever, cough, a runny nose, red and watery eyes, and a distinctive red, blotchy rash that usually begins on the face and spreads downward. The virus spreads through the air when an infected person coughs or sneezes and can lead to serious complications such as pneumonia or brain inflammation. Despite its severity, measles is preventable through a safe and effective vaccine, as per the Mayo Clinic.
How Contagious Is Measles?
Measles is among the most contagious diseases in the world. The virus spreads through airborne droplets that can linger in the air or on surfaces for hours. Up to 90% of unvaccinated people who are exposed to measles will become infected. A single infected person can pass the virus to an estimated 12 to 18 others through close contact or shared spaces. People can transmit the virus days before symptoms become obvious and continue spreading it after the rash appears, according to the World Health Organization.
How Long Is Someone Contagious With Measles?
Someone infected with measles can spread the virus from four days before the rash develops to four days after it appears. The virus spreads so efficiently that about 90% of people who are unvaccinated or have never had measles will become infected after being exposed.
In November, Canada lost its measles elimination status following a significant outbreak, according to the Pan American Health Organization, which works closely with the World Health Organization.
“It’s important to say that all the other 34 countries in the region, they keep their certification as measles-free,” said PAHO/WHO Director Dr. Jarbas Barbosa at the time, as per NPR News.
U.S. health officials have also warned that genetic links between outbreaks in different states suggest continued spread.
“The trajectory that we’re looking at now is that we do anticipate more cases well into January,” Bell said. “What that means for us nationally in terms of how they are defining our designation in this country as having eliminated measles is unclear.”
© 2024 Bennett, Coleman & Company Limited

