- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Trump Administration Reclassifies Marijuana As A Schedule 3 Drug, Moving It Closer To Prescription Painkillers

Credits: Canva
President Donald Trump on Thursday signed an executive order aimed at speeding up the reclassification of cannabis, a move that would allow the Food and Drug Administration to more closely examine its potential medical uses. The order states that the administration’s policy is to expand research on medical marijuana and CBD so patients and doctors have clearer, evidence-based guidance.
It also stresses the need to narrow the gap between widespread use and limited scientific understanding of possible benefits and risks, according to NBC News. Under the proposed change, cannabis would be moved to Schedule III, placing it in the same category as certain commonly prescribed pain medicines, including Tylenol with codeine.
Trump Signs Order to Ease Restrictions on Marijuana
President Donald Trump has signed an executive order that marks one of the most notable shifts in U.S. marijuana policy in decades. On December 18, he instructed federal agencies to stop treating marijuana as a Schedule I substance, a category reserved for drugs considered highly dangerous, such as heroin, LSD, and ecstasy.
The Drug Enforcement Administration defines Schedule I drugs as having no accepted medical use and a high likelihood of abuse. Trump directed that marijuana instead be placed under Schedule III, which the DEA describes as substances with recognized medical uses and a moderate to low risk of physical or psychological dependence. If implemented, this change would move marijuana away from the same legal framework as heroin and LSD and align it more closely with medicines that have established therapeutic value, such as certain acetaminophen and codeine combinations.
What Is A Schedule 3 Drug?
Schedule III drugs are regulated substances that are permitted for specific medical purposes under federal law. While their manufacture, distribution, and use are tightly controlled, they can be prescribed by licensed healthcare professionals. These rules also spell out penalties for illegal trafficking. Examples of Schedule III drugs include ketamine, anabolic steroids, and some acetaminophen-codeine medications.
Marijuana has long been listed as a Schedule I drug, a classification that assumes it is highly dangerous, addictive, and lacking medical value. Reclassifying it would shift cannabis into a category that allows lawful medical prescribing.
Marijuana has remained a Schedule I substance since the passage of the Controlled Substances Act in 1970, according to CBS News.
What All Medicines Are Included In Schedule 3 Drug?
In the United States, Schedule III drugs are recognized for medical use and are considered less likely to be abused than Schedule I or II substances, though they may still cause moderate physical or high psychological dependence. This group includes anabolic steroids, ketamine, certain opioid combinations containing codeine or hydrocodone, such as Tylenol with Codeine, and barbiturates like pentobarbital. These medications are subject to strict rules around prescribing, dispensing, and storage to balance their medical benefits with the risk of misuse.
FDA To Study Marijuana's Medicinal Properties
Once the reclassification is finalized, it is expected to make research easier by reducing funding and regulatory barriers for clinical trials. Pharmaceutical companies would also find it simpler to seek FDA approval for cannabis-based medicines. Because marijuana has been listed as Schedule I, many drug makers have avoided pursuing trials due to heavy bureaucracy and high costs. Moving it to Schedule III would lower these hurdles and allow the FDA to properly study its medical potential. This could eventually expand access to cannabis-based treatments for groups such as seniors and veterans, regardless of differing state laws.
Although the change would not legalize marijuana outright, it could bring meaningful practical effects, including clearer medical access and fewer legal uncertainties for consumers and businesses. Trump also made clear that he does not support recreational legalization. He warned that using powerful controlled substances for non-medical reasons is unsafe and said that unless a doctor recommends a drug for medical purposes, people should avoid using it, according to NBC News.
James Van Der Beek: What Kind Of Cancer Has The Actor Been Diagnosed With?

Credits: Canva
More than a year after Dawson’s Creek star James Van Der Beek revealed that he had been diagnosed with stage 3 colorectal cancer, the actor has shared a new update on how the illness has changed his outlook on life in unexpected ways. Speaking to host Craig Melvin on the December 19 episode of Today, James reflected on the moment he first heard the diagnosis.
“As soon as I found out, I remember thinking, ‘This might end up being the best thing that ever happened to me,’” he said. “There was this quiet voice in my head telling me that this diagnosis would push me to make changes I would never have made otherwise.”
As James Van Der Beek opens up about his health journey, many are asking: what kind of cancer was he diagnosed with?
James Van Der Beek Cancer: What Kind Of Cancer Has He Been Diagnosed With?
James Van Der Beek was diagnosed with stage 3 colorectal cancer after he began noticing changes in his bowel habits in the summer of 2023. At first, he brushed off the symptoms, assuming they were linked to his coffee intake. However, when the changes did not go away, he decided to consult a doctor.
A colonoscopy later confirmed the cancer diagnosis. The news came as a shock, especially since Van Der Beek had no known family history of colorectal cancer and believed he was in excellent health due to his active lifestyle and balanced diet.
What Is Colorectal Cancer?
Colorectal cancer develops in the colon or rectum and often begins as small, non-cancerous growths known as polyps. Over time, some of these polyps can become cancerous, interfering with digestion and the body’s ability to process waste.
It is one of the more common forms of cancer and can be difficult to detect early because symptoms may not appear right away. When they do, they often include blood in the stool, persistent changes in bowel habits, abdominal discomfort, and unexplained weight loss. According to the Mayo Clinic, early screening plays a critical role in detecting the disease when it is most treatable, and lifestyle choices can significantly influence risk and outcomes.
James Van Der Beek Cancer: Regular Screenings and Colonoscopies
As colorectal cancer often shows no symptoms in its early stages, routine screening is essential. Colonoscopies allow doctors to spot and remove precancerous polyps and detect cancer before it spreads. Early intervention has been shown to lower both the number of cases and deaths associated with the disease.
Data from the CDC highlights the importance of early detection, with survival rates varying widely by stage. While stage I colorectal cancer has a five-year survival rate of about 91 percent, that number drops sharply to around 14 percent for stage IV cases, according to the American Cancer Society. Health experts recommend beginning regular screening at age 45, or earlier for people with higher risk factors. Colonoscopy remains the most effective screening tool, as it examines the entire colon and allows for immediate removal of suspicious growths.
James Van Der Beek Cancer: What Was The Prognosis?
James Van Der Beek’s outlook following his stage 3 colorectal cancer diagnosis has been described as cautiously hopeful. His cancer was identified while still localized, a category associated with a significantly higher survival rate. According to the American Cancer Society, localized colorectal cancer has a five-year survival rate of approximately 91 percent.
James Van Der Beek Cancer: What Treatments Did James Undergo?
James Van Der Beek has chosen not to share detailed information about the specific treatments he has received for his stage 3 colorectal cancer. In general, treatment for this stage of the disease typically involves surgery followed by chemotherapy, and in some cases, radiation therapy.
Nutritional support also plays an important role, particularly because colorectal cancer and its treatments can affect digestion. While Van Der Beek has kept the details private, he has emphasized that he is actively addressing his diagnosis and prioritizing his overall health as part of his recovery.
Did Scientists Just Come Up With A New Blood Test That Detects And Monitors Lung Cancers In Real Time?
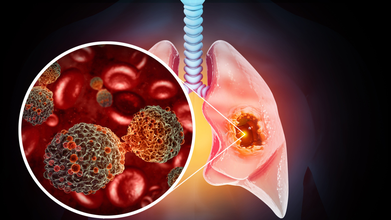
Credits: iStock
A team of researchers developed a novel blood test that could detect and monitor lung cancer in real time. They used the technique Fourier Transform Infrared (FT-IR) micro spectroscopy to detect a single lung cancer cell in a patient's blood. This technique combines advanced infrared scanning technology with computer analysis and focuses on the unique chemical fingerprint of cancer cells. The research has been lead by the team from University Hospitals of North Midlands NHS Trust or UHNM, Keele University, and Loughborough University.
Study's lead author Professor Josep Sulé-Suso, Associate Specialist in Oncology at UHNM, says, "This approach has the potential to help patients receive earlier diagnoses, personalized treatments, and fewer invasive procedures, and it could eventually be applied to many types of cancer beyond lung cancer."
How Does This New Blood Test Work?
Circulating tumor cells or CTCs are a type of cancer cell that can break away from a tumor and travel in bloodstream. They can also provide information on how the disease progresses and how well does a treatment work. CTCs are also the cells that leads to spread of cancer or what is known as metastases.
How is it novel and differ to the current methods in use? Current methods detect CTCs, but use a very complicated, expensive and time-consuming procedure. The current methods could also sometimes miss cancer cells altogether, as the cells often change their characteristics while circulating in the blood.
The researchers identify circulating tumor cells, or CTCs, in blood samples by directing a powerful infrared beam at them, similar in principle to the light used in a TV remote but much stronger.
Because different chemicals absorb infrared light in unique ways, CTCs produce a distinctive absorption signature, often described as a chemical fingerprint. Advanced computer analysis of this data can quickly determine whether tumor cells are present in the bloodstream.
Published in the journal Applied Spectroscopy, the method is both simpler and more cost-effective than current techniques. It uses standard glass slides already common in pathology laboratories, which could make it easier to incorporate into routine clinical use.
The team plans to test the approach in larger patient populations, with the goal of developing a fast, automated blood test that can be seamlessly added to cancer care pathways.
How Common Is Lung Cancer?
As per the World Cancer Research Fund, there were 2,480,675 new cases of lung cancer in 2022. The numbers have projected to rise significantly by 2050. Key drivers of lung cancer include tobacco usage, air pollution, and occupational exposure. There has also been a rise in lung cancer rates in women and seen disparities across regions.
What happens in lung cancer? The National Health Institute (NIH) notes that lung cancer refers to tumors originating in the lung parenchyma or within the bronchi. This is the third most common cancer in the US. Your cells divide and make more copies of themselves as a part of their normal function, however, they get mutations that cause them to keep making more of themselves when they should not. This is how damaged cells divide at an uncontrollable rate and create masses or tumor that keep your organs from working properly.
Epstein Files Photos Show A Bottle Of Phenazopyridine, Why We Think This UTI Medication Was There
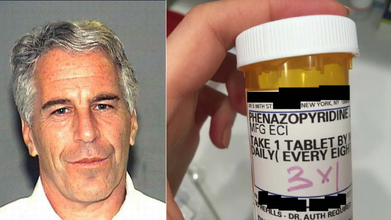
Credits: Wikimedia Commons and House Oversight Committee
Epstein Files photos show someone holding a bottle of medication, which has come up in the latest batch of photos released on December 18 by House Oversight Democrats, as part of the 68 photos from Jeffrey Epstein's estate. On the bottle is a label with the word "Phenazopyridine". Why was this medicine there? What does it do?
What Is Phenazopyridine And Why We Think It Was Found At Epstein's House
Phenazopyridine is commonly used to reduce symptoms like urinary pain, burning, or feeling the urgency to pee. It is also used to treat bladder infections or urinary tract infection. WebMD says that it could also be used to help relieve symptoms from other causes like surgery or another procedure.
The Epstein files as we know are a body of documents that detail the criminal activities of American financier and convicted child sec offender, previously it had been reported that he allegedly paid doctors to medicate underage abuse victims. He would get doctors to prescribed anti-anxiety or anti-depressants medications, birth control pills, and also got the victims screened for sexually transmitted diseases, as HuffPost reports.
This is why we think he may have used this common UTI medication: Urinary Tract Infections or UTI are very common after sexual activities, especially for women. It is also because intercourse could push bacteria like E.coli from the genital area into the urethra, this could lead to infection. Since the urethra is shorter in women, it makes it easier for bacteria to reach the bladder. Furthermore, Phenazopyridine is also an over-the-counter, non-antibiotic method to prevent or reduce post-coital UTI frequency and could also eliminate the need for antibiotics.
Epstein Used Medicine To Drug His Victims
Previous reports too show how Epstein had used medicines to drug his victims. Virginia Giuffre told the Miami Herald that she was only 16 when she was recruited to give Epstein "massages". She revealed that she was prescribed Xanax, when she lived and traveled with Epstein for several years.
Another accuser Sarah Ransome said that when she told a doctor that she was sexually abused by Epstein, she was prescribed lithium.
What Do These Medicines Do To You?
Alprazolam, commonly sold under the brand name Xanax is used for anxiety and panic disorder. WebMD says that it produces a calming effect on the brain and helps reduce anxiety, while promoting relaxation. Reports show that he got a doctor prescribe these drugs so he would control the young women, and sedate them and induce amnesia of the abuse. Experts have noted that it can be used as a "date rape" or "grooming drug" because it makes users "zombie-like" or "dazed".
Lithium on the other hand, notes the National Health Service or NHS, UK, slows down the thinking and makes the people feel a bit "numb". Other side effects of lithium also include feeling tired or sleepy, along with lightheadedness, drowsiness, confusion, blackout, and difficulty speaking. This could also be used to control young victims.
Note: No reports have confirmed the use of Phenazopyridine found in Epstein estate as of now. This piece will be updated as and when there is any other information.
© 2024 Bennett, Coleman & Company Limited

