- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
TrumpRx Is Live: How Is It Changing US Healthcare System? Explained

Credits: Wikimedia Commons and Canva
TrumpRx is live. President Donald Trump on Thursday announced the launch of TrumpRx, a direct-to-customer website that will lower prescription drug costs in the US.
As per the president, millions of Americans would save money through this platform. However, it is still unclear if all patients, especially those with insurance coverage will see more cost saving while using this website to buy their medicines.
TrumpRx is for people willing to pay cash and forgo insurance, which means people without or with limited coverage could benefit.
"You are going to save a fortune and this is also so good for overall health care," said Trump at the TrumpRx launching night on Thursday.
TrumpRx Is Live: How Does It Work?

TrumpRx does not sell drugs directly to American patients, rather acts as a central hub that points them to drugmakers who are offering discounts. For instance, Eli Lilly and Novo Nordisk were already offering their popular weight loss drugs at hefty discounts, TrumpRx would further give them discount coupons and redirect them to their website.
This means if someone clicks on Eli Lilly's Zepbound offer, it will send them directly to the company's platform, where the person must submit prescription details.
TrumpRx Is Live: Which Drugs Are Now Cheaper?
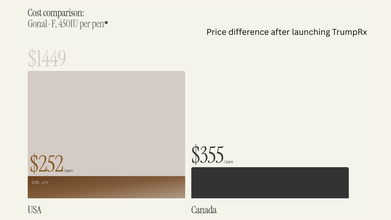
At launch, the site only featured medications from five companies, which are:
- AztraZeneca
- Lilly
- EMD Serono
- Novo Nordisk
- Pfizer
However, as per a White House fact sheet, TrumpRx will list drugs from other companies "in coming months".
TrumpRx marks the government’s latest attempt to curb soaring prescription drug prices in the US, which are on average two to three times higher than in other developed countries and can be up to 10 times costlier than in some nations, according to public policy think tank Rand Corp.
TrumpRx Is Live: Is It Really Beneficial?
TrumpRx “doesn’t appear to be a one-stop solution” to high drug costs for most Americans, said Juliette Cubanski, deputy director of the Program on Medicare Policy at KFF, a health policy research organization. While cash-pay options may offer better value for people without insurance, it remains unclear how many individuals would actually benefit from TrumpRx, she noted.
“If someone can already access a medication through their insurance with a relatively affordable copay, there isn’t much added advantage to using the TrumpRx website,” Cubanski said.
She also pointed out that insured consumers who purchase medicines through direct-to-consumer platforms may find those purchases do not count toward their insurance benefits, meaning they do not help meet deductibles or out-of-pocket maximums.
At the same time, Cubanski said TrumpRx could play a role in improving access to certain drugs at lower prices, especially medications that are not widely covered by insurance in the US, such as obesity treatments. Medicare is set to cover weight-loss drugs for the first time later this year under agreements struck by Eli Lilly and Novo Nordisk with Trump, though many employers remain reluctant to include these medicines in their coverage.
However, she added that many other drugs expected to be offered on TrumpRx are already commonly covered by insurance, and several are also available as lower-cost generics from rival manufacturers.
Cheaper Copy Of Wegovy Pill By Hims & Hers Now Available For 49, Novo Nordisk Says It Will Take Legal Actions

Credits: iStock
Cheaper copy of Wegovy by Hims & Hers for $49 has drawn tensions, with Novo Nordisk now approaching to take legal actions. The pill by Novo Nordisk is sold for $149. In a statement, Novo said, "The action by Hims & Hers is illegal mass compounding that poses a significant risk to patient safety. Novo Nordisk confirmed that the company will take legal and regulatory actions to protect patients.
“Novo Nordisk will take legal and regulatory action to protect patients, our intellectual property and the integrity of the US gold-standard drug approval framework. This is another example of Hims & Hers’ historic behaviour of duping the American public with knock-off GLP-1 products, and the FDA has previously warned them about their deceptive advertising of GLP-1 knock-offs,” the statement said.
Read: Wegovy Pills Now Available At Your Pharmacies, Here's What To Know About Its Usage
Cheaper Copy Of Wegovy Pill: What Unveiled Afterwards?
After the cheaper copy of the pill was launched by Hims & Hers, shares of Novo Nordisk and rival Eli Lilly fell by 7%. Whereas, stock for Hims & Hers initially spiked after the announcement, but pared gains after Novo said that it would fight the rollout.
Hims & Hers was previously offering compounded semaglutide, the active ingredient in Novo Nordisk's popular weight loss drug Ozempic and Wegovy, in injectable format. The company has now extended to offer the oral version.
Cheaper Copy Of Wegovy Pill: How Much Does This Cost?
Hims & Hers said that the Wegovy copy pill cost $49 for the first month and $99 with a 5-month plan. While semaglutide's patent is protected in the US until 2032, however, Hims & Hers claim that the copies are 'personalized' and therefore legal.
“This compounded product uses a different formulation and delivery system than FDA-approved oral semaglutide,” Hims & Hers said. “This once-a-day pill has the same active ingredient as Wegovy and empowers providers to tailor treatment plans specifically for those who prefer to avoid needles or need smaller doses to help to balance side-effects,” it said.
Cheaper Copy of Wegovy Pill: Novo Nordisk Rejects Hims & Hers Claims
Novo Nordisk however, highlighted that it manufactures Wegovy pill by using SNAC technology. This technology helps in absorption when administered orally. Therefore, it is not clear how Hims & Hers copy formula could match that level of absorption.
Last year, Novo Nordisk and Hims & Hers partnered to offer weight loss jabs at a discounted price to telehealth company's customer. However, Novo Nordisk ended the collaboration just two months later, stating that Hims & Hers used 'deceptive' marketing that put patient safety at risk
Cheaper Copy of Wegovy Pill: Does Eli Lilly Have A Weight Loss Pill Yet?
While rival Eli Lilly does not have the weight loss pill yet, it is expected to launch orforglipron in the first half of this year. The pill is currently pending Food and Drug Administration approval.
Read: Eli Lilly Sends Weight-Loss Pill For Approval: Is Oral GLP-1 As Effective As The Injections?
“With the ... current legal backdrop, there is no reason why HIMS shouldn’t evaluate these launches for every subsequent weight loss product as the market continues to evolve,” Leerink analyst Michael Cherny said in a note to clients.
Nipah Virus Survivor Speaks Out, Calls for More Awareness

Credit: Canva
The Nipah virus outbreak began in West Bengal, India with two hospital nurses at AIIMS, Kolkata, testing positive for the infection and being quarantined, prompting widespread testing. Soon after, five cases, including a doctor and a staff member, were confirmed and over 100 people were quarantined.
However, one of the nurses, a 25-year-old unidentified man has now made a recovery and revealed his experience with the virus, claiming that despite irritation in the throat and uncertainty about what lay ahead, he had faith in his doctors and fellow nurses.
In an interview with the Metro, he said: “After I was taken off ventilation and regained consciousness, I came to know that I have Nipah. I still had the tube in my mouth, and there was irritation. Despite the irritation and my fear, I had faith in the doctors and nurses.
“I have suffered and I know the symptoms. I will tell people when they should get checked for the Nipah virus. I want to raise awareness about the virus and its symptoms.
“I am not sure how I came in contact with the deadly virus. Maybe it was while treating a patient. But I will continue to work as a nurse. I am waiting to rejoin the hospital,” he added.
The unidentified healthcare professional remains very weak physically and is undergoing physiotherapy to regain his strength. “I was bedridden for over a month. I am still very weak and have an unstable gait. So, I am undergoing physiotherapy,” he said.
The other nurse, a woman, remains in a coma but has been taken off ventilation support, a hospital official confirmed this week. .
Nipah Virus: What Is It And What Are Its Symptoms?
According to WHO, Nipah virus is a zoonotic illness which means it is mostly transmitted from animals to humans through bats. However, it can also spread through fruits that have been contaminated by the saliva, urine or droppings of infected bats. Human-to-human transmission can also occur through close contact with an infected person or their bodily fluids.
The illness has a 75 percent fatality rate and there are no vaccines to protect the public.
The virus was first identified in 1998 during an outbreak among pig farmers in Malaysia and soon made its way to India and Bangladesh in 2001 with cases often involving family members or caregivers tending to infected patient.
READ MORE: Nipah Virus Outbreak In India: Myanmar Airport Tightens Health Screenings
Although Nipah virus has caused only a few known outbreaks in Asia, it infects a wide range of animals and causes severe disease and death in people. Some of its common symptoms include:
- Fever
- Headache
- Breathing difficulties
- Cough and sore throat
- Diarrhea
- Vomiting
- Muscle pain and severe weakness
Samples collected from the patient’s home and workplaces, including pets and partially eaten fruits dropped by bats, all tested negative for the virus, and the exact source of the infection could not be identified.
What Does WHO Say?
The World Health Organization has declared it is unlikely India's deadly Nipah Virus outbreak will cross borders and reach other nations, noting that countries do not need to set any travel restrictions in place.
In an email to Reuters, officials said: "The WHO considers the risk of further spread of infection from these two cases is low". adding that India has the capacity to contain such outbreaks.
"There is no evidence yet of increased human to human transmission," it said, adding that it has coordinated with Indian health authorities.
While health officials state it is nearly impossible for the virus to transmit across countries and unlikely to cause an international outbreak, countries including Australia, Vietnam, Thailand, Malaysia and Singapore continue to remain on high alert and have begun airport screenings.
Ahmedabad Toddler Swallows Hulk Toy, Showed X-Ray, Doctors Remove It Via Endoscopy
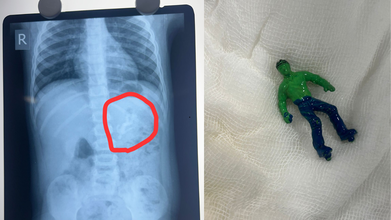
Credits: X
Ahmedabad toddler, one-and-a-half-year-old boy swallowed a 'Hulk' toy, which is based on a popular comic superhero. The toy was stuck in his stomach when his parents took him to the Civil Hospital. According to reports by News18, the child is identified as Vansh who showed the signs of discomfort and began vomiting. This is what alarmed the parents.
As per the News18 report, his mother Bhavika was suspicious when she noticed that one of his toys was missing. The child was rushed to the hospital and an X-ray revealed that he had swallowed the entire plastic toy. The toy was not broken.
Hindustan Times reported that Dr Rakesh Joshi, Head of the Department of Pediatric Surgery removed the toy through upper GI endoscopy. "Had it been a little late, the toy could have moved further from the stomach and got stuck in the intestines. In that case, there would have been a risk of intestinal blockage and even rupture," the senior doctor said.
"There is a natural valve between the esophagus and the stomach. The biggest challenge was to take out a whole toy through this valve. When we tried to grab it with the endoscope, the toy kept slipping because of the air in the stomach. Pulling the toy by its hand or foot raised the possibility of it getting stuck in the valve and causing it permanent damage," he said.
The doctor noted that if the toy had further slipped down, it would have increased the risk of intestine rupturing.
Ahmedabad Toddler Swallows Hulk Toy: What Parents Must Keep In Their Mind
Under the Toys (Quality Control) Order, 2020 issued by the Department for Promotion of Industry and Internal Trade under the Ministry of Commerce and Industry, toy safety in India was brought under mandatory BIS certification from September 1, 2020. The move aims to ensure safer toys for children while also supporting the government’s policy of curbing non-essential imports.
Industry sources estimate that more than 85 percent of toys sold in India are imported. Officials say the Toys Quality Control Order is a key step in preventing the entry of cheap and substandard toys into the domestic market, many of which fail to meet basic safety requirements.
Ahmedabad Toddler Swallows Hulk Toy: Standards for Electric and Non-Electric Toys
The quality control order clearly defines safety standards based on the type of toy. Non-electric toys such as dolls, rattles, puzzles, and board games must comply with IS 9873 (Part 1):2019. These toys do not rely on electricity for any of their functions.
Electric toys, which include at least one function powered by electricity, are required to meet the standards outlined under IS 15644:2006. Compliance with these standards is mandatory before such toys can be sold in the Indian market.
Ahmedabad Toddler Swallows Hulk Toy: Risks Linked to Untested Toys
Toys that are not tested by NABL-accredited toy testing laboratories can pose serious health risks to children. Sharp edges and poorly finished parts can cause cuts and injuries. PVC toys may contain phthalates, which are considered harmful chemicals.
Many low-quality toys have also been found to contain lead, a substance known to be particularly damaging to brain development in children. Soft toys with fur or hair can trigger allergies or become choking hazards. In some cases, small body parts can get stuck in gaps or holes, increasing the risk of injury.
Testing by NABL-accredited laboratories ensures that toys are safe, durable, and suitable for specific age groups. Parents are advised to check for IS marks on toys before purchasing, as this indicates compliance with Indian safety standards.
Ahmedabad Toddler Swallows Hulk Toy: What Parents Should Check Before Buying Toys
Experts recommend avoiding toys with small detachable parts for toddlers and young children, as they are more likely to put objects in their mouths. Toys should always match the child’s age, skill level, and interests.
Parents are also urged to look for IS marks, which confirm that the toy has been tested and certified. Loud toys should be avoided, as prolonged exposure to sounds above 85 decibels can harm a child’s hearing.
Electric toys with heating elements should be used with caution or avoided altogether due to burn risks. Finally, toys with sharp edges or shooting components should be carefully examined to prevent cuts and injuries.
© 2024 Bennett, Coleman & Company Limited

