- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Ultra-Low-Dose CT Scans May Help Early Detection Of Pneumonia
Credit: Canva
Low-dose CT chest scans could help detect pneumonia in at-risk patients while exposing them to only small amounts of radiation, a new study has found. The research, published in Radiology: Cardiothoracic Imaging, shows that ultra-low-dose scans can effectively detect pneumonia in patients with compromised immune systems, enabling doctors to treat the infection before it becomes life-threatening. According to the researchers, these scans expose patients to just 2% of the radiation dose used in a standard CT scan.
"This study paves the way for safer, AI-driven imaging that reduces radiation exposure while preserving diagnostic accuracy,” lead researcher Dr Maximiliano Klug, a radiologist with the Sheba Medical Center in Ramat Gan, Israel, said in a news release. He added that CT scans are the gold standard for detecting pneumonia but there are concerns regarding the risk posed by repeated exposure to radiation. There is a solution- ultra-low-dose CT scan. However, the problem is that these scans can be grainy and hard to read, researchers said.
Study Gives Solution To This
To overcome that, Klug's team developed an AI program that could help "de-noise" low-dose scans, making them sharper and easier to read. Between September 2020 and December 2022, 54 patients with compromised immune systems who had fevers underwent a pair of chest CT scans -- a normal dose scan and an ultra-low-dose scan. The AI program cleaned up the low-dose scan, and then both sets of images were given to a pair of radiologists for assessment. Radiologists had 100% accuracy in detecting pneumonia and other lung problems with the AI-cleaned low-dose scans, but 91% to 98% accuracy in examining the scans that hadn’t been improved through AI, results show.
"This pilot study identified infection with a fraction of the radiation dose," Klug said. "This approach could drive larger studies and ultimately reshape clinical guidelines, making denoised ultra-low dose CT the new standard for young immunocompromised patients.
How Can You Detect Pneumonia?
Pneumonia is a lung infection that causes the air sacs in the lungs to fill with fluid or pus and can be caused by bacteria, viruses, or fungi. The symptoms can range from milk to severe, which includes:
Coughing with or without cough
Fever
Chills
Trouble breathing
Chest pain, especially when breathing deeply or coughing
Sweating or chills
Rapid heart rate
Loss of appetite
Bluish skin, lips, and nails
Confusion.
How to detect Pneumonia in coughing newborns and toddlers?
Pneumonia can severely affect newborns and young children as their lungs are comparatively more sensitive. As per Dr Goyal, young children can cough for various reasons including seasonal infections and tonsillitis, which is very common in this age group. But if they look visibly irritable and have poor sleep patterns, then parents must reach out to an expert. "I am not saying that parents must visit a hospital but any local paediatrician would be able to detect pneumonia in your kid.
Catherine O'Hara Cause Of Death Is Pulmonary Embolism; She Also Had Rectal Cancer

Credits: Wikimedia Commons
Catherine O'Hara's cause of death is pulmonary embolism, confirmed medical examiner. She died on January 30 in a Los Angeles hospital, at the age of 71. The Schitt's Creek star also had blood clot in her lungs and her death certificate also listed rectal cancer as the long term cause of death.
She had been receiving treatment for the cancer since March 2025.
Catherine O'Hara Cause Of Death: What Is Pulmonary Embolism?
As per the National Health Service (NHS), a pulmonary embolism is a life-threatening condition that happens when a blood vessel in the lungs is blocked by a blood clot.
The common symptoms may include:
- Difficulty in breathing
- Chest pain
- Cough up blood
The blood clot starts in a deep vein in the leg and travels to the lung in most cases. Rarely, the clot forms in a vein in another part of the body, noted Mayo Clinic. When a blood clot forms in one or more of the deep veins in the body, it is called a deep vein thrombosis or DVT.
Other symptoms of pulmonary embolism include:
- irregular heartbeat
- lightheadedness or dizziness
- excessive sweating
- fever
- leg pain or swelling, usually at the back of lower leg
- clammy or discolored skin
As per Cleveland Clinic, about a third of people with a pulmonary embolism die before diagnosis and treatment, highlighting the condition's severity.
Catherine O'Hara Cause Of Death: She Also Had Rectal Cancer
Catherine O'Hara also was battling rectal cancer, which had been a long-term health challenge for her. The diagnosis was kept private. As per TMZ and The Associated Press, the diagnosis was kept private with only close family and her medical team aware of the details. Her struggle with cancer was not widely known, which made her news of rectal cancer more shocking to her fans and many in the industry.
Rectal cancer is a serious and often aggressive disease. According to the Mayo Clinic and the American Cancer Society, it typically starts as abnormal growths known as polyps in the rectum and can require intensive treatment, including surgery, radiation, and chemotherapy. Colorectal cancer—which includes cancers of both the colon and rectum—remains one of the leading causes of cancer-related deaths in the United States. This year, nearly 50,000 people are expected to be diagnosed with rectal cancer, while colorectal cancer overall is projected to claim around 55,000 lives nationwide.
Rectal cancer too can increase the risk of developing blood clots, making complications like pulmonary embolism, more common among cancer patients.
Catherine O'Hara Cause Of Death: She Was Born With A Rare Genetic Condition
Speaking in previous interviews, O'Hara revealed that she was born with a rare genetic condition called situs inversus. This means the organs are mirrored from their usual positions. Her heart, for instances, pointed to the right side of her chest, a condition known as dextrocardia. While this is a harmless condition, situs inversus could sometimes be associated with other complications.
ER Patients in Massachusetts Wait 3+ Hours on Average, Third-Longest in the US

Credits: Canva
ER Patients in Massachusetts Wait: Any visit to hospital must be treat with uttermost urgency, especially when it is the visit to the ER. What is worse is waiting a long time to see a doctor or a medical team even during an ER visit. A new analysis by health insurance experts at Compare the Market finds that the US has the lowest number of hospitals, per capita, in the world, which means 1.84 hospitals per 100,000 citizens. This is what makes the waiting time longer.
This has placed US behind countries like Chile which has 1.85 hospitals per 100,000 citizens and Thailand with 1.89 hospitals per 100,000 citizens. According to the same analysis, South Korea ranks top with 7.38 hospitals per 100,000 citizens, followed with Japan with 6.64 hospitals per 100,000 citizens. Among the 54 countries evaluated in the analysis, the United States ranked 29.
ER Patients in Massachusetts: Longest Wait Hours In US
| US States | ER Wait Time (in minutes) |
| Maryland | 228 |
| Delaware | 195 |
| Massachusetts | 189 |
| Rhode Island | 185 |
| New York | 184 |
| Arizona | 176 |
| New Jersey | 173 |
| Connecticut | 166 |
| California | 164 |
| Illinois | 157 |
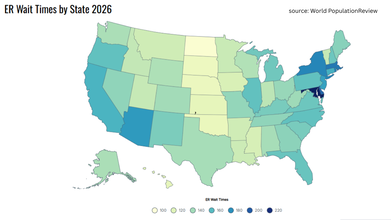
The top on the list was Maryland with 228 minutes, or almost 4 hours, followed by Delaware with 195 minutes.
ER Patients in Massachusetts: Long Wait Hours Leads To Death
There have been cases where long ER waits have led to patients' deaths in the US. A KKTV report from 2022 notes a 77-year-old North Carolina patient who went to New Hanover Regional Medical Center waited for more than 5 hours. The patient met with a triage at 8.43pm and was given an urgent designation, but was told to sit in the waiting room. Investigation revealed that she was not assessed until the next day at 8.am for her vitals to be taken. The woman was pronounced dead at 4.25am.
A Fortune report from 2025 interviewed an Illinois family who lost their father Bill Speer to ER boarding. Tracy Balhan, the daughter revealed that her father died after struggling with dementia. She took him to the emergency room at Endeavor Health's Edward Hospital in Chicago suburb of Naperville. However, Speer spent 12 hours in the ER.
An older report by EMS World from 2011, revealed a case of a Texas man who died after waiting for 16 hours for treatment in ER.
This is not just the case in the US, but a very recent case reported by Health and Me was of a 44-year-old Indian origin man, Prashant Sreekumar, who died at a Canadian hospital's ER after an 8-hour weight. His father, Kumar Sreekumar told the Global News that he was checked in at triage and then seated in the waiting room. When his father reached the hospital, he told him, "Papa, I cannot bear the pain." "It went up, up, and up. To me, it was through the roof," his father said. He was finally called for treatment after more than eight hours of wait." After sitting maybe 10 seconds, he looked at me, he got up and put his hand on his chest and just crashed," his father said.
ER Patients in Massachusetts: What Is ER Boarding?
Read: “Papa, I Can’t Bear the Pain”, Says Indian-Origin Man Who Dies After Eight-Hour ER Wait in Canada
ER boarding is a common term that is used to highlight the health care system's struggle which includes shrinking points of entry for patients seeking care outside of ERs and hospitals prioritizing beds for procedures insurance companies often pay more for, and for making patients wait long hours for ER visits.
1 in 6 visits to the ER in 2022 that resulted in hospital admission had a wait of four or more hours, as per an Associated Press and Side Effects Public Media data analysis.
Wegovy Pill: FDA Says The TV Ad By Novo Nordisk On Its Weight Loss Pills Are Misleading

Credits: Canva
Wegovy pill by Novo Nordisk is now under scrutiny after the US Food and Drug Administration (FDA) raised concerns over a television advertisement. FDA said that the commercial includes "false and misleading" claims about the obesity drug's benefits.
In a letter (reported first by Bloomberg) dated February 5, the FDA said the advertisement misbrands the oral weight loss drug. FDA said that it made its distribution a potential violation of federal law. FDA has asked the Danish drugmaker to take immediate corrective action, which include:
- Pulling the ad
- Revising all advertisements that contain misleading statements
Wegovy Pill: FDA Questions Claims of Superiority and Emotional Benefits
As per the letter, the FDA found that the ad misleadingly suggests the Wegovy pill offers superior benefits when compared to other approved GLP-1 weight loss drugs. The regulator flagged phrases such as “live lighter” and “a way forward,” saying they imply greater weight loss and added advantages without evidence to support those claims.
The FDA also took issue with what it describes as emotional and psychological messaging, saying that the ad appears to imply benefits beyond physical weight loss, including emotional relief, reduced psychological burden, hope, or direction in life. FDA notes that this claim positions the drug as a solution to broader life challenges rather than just a treatment to obesity.
Furthermore, FDA said that the ad failed to adequately present risk information in both audio and text formats, which is mandatory requirement for television drug advertising.
Wegovy Pill: Novo Nordisk Responds To FDA Notice
Novo Nordisk confirmed on Monday that it has received the FDA’s letter. The company clarified that while the advertisement has been running since the pill’s launch, it is not linked to its Super Bowl advertising.
“We take all regulatory feedback seriously and are in the process of responding to the FDA to address their concerns regarding the advertisement’s presentation,” said Liz Skrbkova, Novo Nordisk’s head of US media and stakeholder relations, in a statement.
Novo Nordisk is already under pressure to defend its position in the weight loss market, as it is facing a stiff competition from rival Eli Lilly. Furthermore, on Monday, Novo also filed a lawsuit against telehealth company Hims & Hers, seeking to block the mass marketing of compounded versions of its Wegovy pill and injections.
Read: Wegovy Pills Now Available At Your Pharmacies, Here's What To Know About Its Usage
Wegovy Pill: How Effective Is It?
After injection by Novo Nordisk, of the same name, Wegovy, which has been on the market since 2021, its popularity grew so much that it was in short supply until February 2025. The pill version has now come out, which many experts believe will expand its accessibility. As the monthly supply of pill is expected to be cheaper than the monthly supply of the weight loss injection.
A study published in the New England Journal of Medicine show that a 25 milligram Wegovy pill led to 13.6% reduction in weight on average over 64 weeks. When compared to placebo, the result was only 2.2% of weight loss. Novo Nordisk says that those who stayed on the treatment and reduced their calorie intake, it would lead to a loss of 16.6% of their weight.
Wegovy Pill: What Are The Side Effects?
Digestive problems such as nausea and vomiting remain the most common side effects of GLP-1 drugs. These issues were also reported in studies of the pill versions. Around 7 percent of participants taking the Wegovy pill stopped treatment because of side effects, compared with 6 percent in the placebo group. In orforglipron’s trial, up to 10 percent of patients discontinued treatment, compared with 3 percent on placebo.
One key difference lies in how the medications are used. The Wegovy pill must be taken on an empty stomach with a small amount of water. Patients are advised not to eat, drink, or take other medicines for at least 30 minutes afterward. Doctors say this requirement has limited the use of Rybelsus, the pill form of semaglutide approved for diabetes, compared with Ozempic.
© 2024 Bennett, Coleman & Company Limited

