- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
US For The First Time Sees A Drop In Obesity Rates

Obesity rates drop in US for the first time in a decade (Credits: Canva)
In over a decade, for the first time, obesity rates in the United States is declining. As per a recent study published last Friday in the journal JAMA Health Forum, it was found that the obesity numbers in the United States have gone down slightly from 46% in 2022 to 45.6% in 2023. While it is only a slight decline, it is also important to note that it is the first drop recorded in almost a decade.
"What we're seeing for the first time is that curve is bending and shows a sign of hope for something that was really a threat to American public health for so many years," said John Brownstein, co-author of the study, who is also the chief innovation officer at Boston Children's Hospital and a professor at Harvard Medical School.
How was the study conducted?
The study reviewed the body mass index (BMIs), a measure of obesity of 16.7 million US adults over a 10-year period. The average BMI rose annually to 30.25, which is considered obese, however, after 2022, a different trend was noticed. It plateaued in 2022, and then dropped to 30.21 in 2023.
Weight Loss Drugs
Brownstein and his team also noted that women and adults aged 66 to 75 saw the greatest drop in obesity rates. Whereas people who live in the South, where there was the highest dispensing rate of weight loss drugs, also known as the GLP-1 receptor agonist, also so a sharp decline in obesity.
Semaglutide is also one of the drugs in the class of the GLP-1 agonists, and also an active agent found in the popular drug like Ozempic and Wegovy. The United States also saw a 700% increase in the use of weight loss drugs, and not as a side-product for diabetes, but specifically for weight loss from 2019 to 2023, mentions a 2024 study titled Shifting Trends in the Indication of Glucagon-like Peptide-1 Receptor Agonist Prescriptions: A Nationwide Analysis, published in the Annals of Internal Medicine. However, these medicines are also used to mainly treat type 2 diabetes or combination of diabetes and obesity.
This is, what Brownstein thinks, has helped a slight decline in the obesity rates in the United States.
ALSO READ: When Ozempic And Wegovy Fail To Work- Why GLP-1 Drugs Aren’t The Magic Bullet For Everyone
What are the other reasons?
Brownstein mentions that there are other reasons too for this slight decline. For instance, looking at the pharmacy prescription, it was seen that while South had the highest dispensing rate of weight loss medication, this area also saw a disproportionately high number of COVID-19 deaths, especially among its obese population.
Another reason, mentions Benjamin Reader, paper's co-author and an assistant professor at Harvard Medical School, is the change in lifestyle. "You have this emergence from COVID, of which people are potentially starting to be more active again, stopping the sedentary habits that they picked up during COVID," said Reader. "All of these forces are at play, and I don't think we can disentangle them from this data."
However, obesity still remains a critical public health issue. Other experts have pointed out that there is a need to look at this positive indicator in a specific database. While this could mean that people are doing better, however, it is also important to see if these factors last long and how the rats play out over time. As of now, as per the Centers for Disease Control and Prevention, nearly 60% of the adults in the United States are obese, and have high blood pressure, while 23% have diabetes.
ALSO CHECK OUT: These Are The Most 10 Obese States In the US
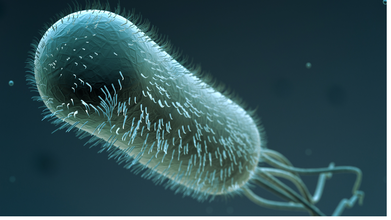
Lancet study shows experimental oral E coli vaccine can help prevent diarrhea in children
Credit: Canva
An experimental oral vaccine has proven to be safe and effective in generating immunity against the Enterotoxigenic Escherichia coli (ETEC), responsible for 75 million diarrhea episodes and over 40,000 deaths annually in children worldwide, according to a new study published in the journal The Lancet Infectious Diseases.
The vaccine ETVAX -- an oral whole-cell vaccine for ETEC -- consists of inactivated E coli bacteria and is designed to prevent bacterial colonization.
In the phase 2 trial, including nearly 5,000 Gambian children aged 6-18 months, ETVAX was well tolerated. There was no increase in the frequency or severity of adverse events, said an international team of researchers, including those from the London School of Hygiene & Tropical Medicine, in the paper.
"Using active and passive surveillance, we confirmed that ETVAX is safe and induces immune responses to colonisation factors and heat-labile toxins," they added.
Produced by ETEC, heat-labile toxins are sensitive to heat and cause watery diarrhea.
What did the study find?
ETVAX showed to be safe, immunogenic, and also offered protection against moderate-to-severe ETEC diarrhea in the presence of co-pathogens.
Importantly, the study provided the first evidence that ETVAX can significantly reduce the incidence of ETEC-positive and all-cause diarrhea, particularly when vaccination is initiated before age 9 months, and in children without concurrent enteroparasitic infections, the team said.
“This study provides the first demonstration of induction of protective efficacy by ETVAX in young children who are at risk,” the researchers said.
“These findings support progression to a large, multi-country, phase 3 trial to confirm ETVAX efficacy against ETEC disease in children and to support ETVAX introduction in high-burden settings,” they added.
These findings support advancing ETVAX to a pivotal phase 3 trial.
How was the study conducted?
The researchers enrolled children ages six to 18 months to receive ETVAX or a placebo at three timepoints (days 1, 15, and 90).
Serious adverse events occurred in 1.0 percent of the ETVAX group and 1.3 percent of the placebo group, with none related to the vaccine.
Among the 122 children in whom immunity was assessed, the ETVAX, developed by Scandinavian Biopharma, increased antibodies to ETEC colonization factors and heat-labile toxins.
What is ETEC?
Enterotoxigenic Escherichia coli (ETEC) is a pathogenic, toxin-producing strain of E. coli that specifically causes watery, non-bloody diarrhea, commonly known as traveler’s diarrhea.
While most E. coli are harmless gut flora, ETEC uses adhesins to colonize the small intestine and release toxins, whereas "generic" E. coli is usually beneficial or benign.
Annually, ETEC causes 220 million diarrhea episodes globally, with 75 million episodes and up to 42,000 deaths in children younger than 5 years, mainly occurring in low-income countries.
Even as climate models predict increased ETEC incidence under warming conditions, the researcher noted that "an ETEC vaccine could reduce illness and deaths, improve child growth, decrease health-care costs, and curb antimicrobial resistance".
Epstein Files Reveal The Doctors Who Helped Keep His Victims Healthy

Credit: Canva
Newly released Department of Justice files have revealed that the convicted child sex offender Jeffrey Epstein and his associates kept a roster of doctors to make sure their victims were tested for STDs, prescribed birth-control pills and inoculated against HPV.
The American serial rapist regularly made payments to at least three New York City gynecologists, a dermatologist and his own personal physician. Apart from New York based doctors, physicians in West Palm Beach, New Mexico, and Ohio, all cities where Epstein had set up residences, have also been named in the Files.
The Epstein Files are over six million pages of documents, images and videos detailing the criminal activities of the financier and his social circle of public figures that included politicians and celebrities.
His co-conspirator Ghislaine Maxwell, who is also a convicted child sex trafficker and sometimes referred to as the "Lady of the House" is serving a 20-year prison sentence at a minimum-security prison camp in Texas.
Who Are The Doctors Named In The Epstein Files?
A December 12, 2012 email shows that an associate whose name is redacted but email address matches to Mark Epstein, Jeffrey’s brother, asks the latter, “Do you remember the name of the Gynocologist [sic] that you used to send your victims to?
“Many years ago you used to send them to a gyno in NY who once commented something to the effect that you were keeping him in business singlehandedly,” the sender continued.
Another 2015 email, when an unidentified person asks which gynecologists Epstein regularly uses for “the girls,” Epstein’s former staff member Bella Klein is seen to write back , “S. Yale and Romoff.” “S. Yale”.
According to The Cut, this may reference to the combined practice of Suzanne Yale, an OB/GYN who shared an office with fellow OB/GYN Adam Romoff in Manhattan for about 45 years. Documents show that Epstein made more than half a dozen direct payments to Romoff and Yale, with the last being on March 14, 2019, four months before he was arrested, for $375 check to Women’s Health of Manhattan, Romoff’s current practice.
Romoff, who still practices his profession, is cited in the emails as the physician for a number of the women associated with Epstein, including Karyna Shuliak, his longtime girlfriend and reported beneficiary of his $100 million fortune. His name shows up in the Epstein files 38 times, though he is never shown to be in direct communication with the abuser himself.
READ MORE: Epstein Files Reveal Secret Muffin Recipe: All You Need To Know
Alexander Shifrin, an OB/GYN and women’s integrative health specialist in Manhattan and Brooklyn is also repeatedly mentioned in the emails and text messages.
Dr Steven Victor, a New York City–based dermatologist has also been mentioned multiple times in the Files. According to a 2012 email revealed in the documents, an unnamed woman who was one of Epstein's "girls" discussed seeing Victor to treat her molloscum contagiosium, a viral skin infection that can be spread through sexual contact.
However, he denies knowing of Epstein's wrongdoings and told The Cut: "Most of the patients referred were adults. There were also some younger patients, including minors. In every such instance, they were accompanied by a legal adult guardian. No patient ever disclosed any inappropriate conduct by Mr. Epstein to me or to my staff. Had anyone done so, I would have immediately reported it to the authorities.
" I am appalled and heartbroken by what Mr. Epstein did to young women and children. My involvement with Mr. Epstein was limited to providing dermatologic care to him and patients referred to my practice. I did not participate in, enable, or have knowledge of any criminal conduct."
Epstein’s own physician, Bruce Moskowitz has also been accused of covering up his sexual activities in 2016. Texts between both men from the year show that Epstein had contracted gonorrhea, an STI transmitted through unprotected vaginal, anal, or oral sex., that year and placed on a rigorous antibiotic course.
In 2018, Epstein reached out to Bruce about two of his "friends" having the same STI. "Think to be safe my two friends should get shot by you tomorrow or send them somewhere close,” he wrote. Moskowitz agreed, proposing a location for him to treat them. “That way I do not have to report the cases to health department including contacts,” he wrote.
Are Any Doctors In The Epstein Files Being Investigated?
While it currently remains unclear whether the physicians were aware of Epstein’s criminal activity, Ohio State University head of gynecology is being investigated after being named in the files for allegedly receiving thousands of dollars in payments for consulting work.
The Files show that Mark Landon, a physician and professor at OSU and the chair of the obstetrics and gynecology department, received about $25,000 quarterly from Epstein in the early 2000s.
Additionally, he also received at least 10 separate payments from Epstein or his associates between June 28, 2001, and April 12, 2005. In an email between Epstein and an attorney he worked with, Darren Indyke, Epstein wrote that they were paying Landon $75,000 a year. The email didn't mention what they were paying Landon for.
In 2006, Indyke wrote to Epstein: "Are we still paying Mark Landon?... Eric was dealing with this, so I am not sure what was decided when the previous payment was made. Landon's agreement requires quarterly payments of $30k to be made to Landon on the 15th of January, April, July and October.
"The previous payment made to Landon was for $25,000 and not $30,000. The contract is terminable at will on 15 days' prior notice. Is NYSG to make payment to Landon by January 15th and if so for $25K or $30K? Please advise."
Records show that Epstein also spent over $200 mailing items to Landon’s Columbus home. It remains unclear what was being mailed to the expert.
READ MORE: Epstein Files Raise Questions About Trump’s Memory Decline
According to a Serena Smith, a spokesperson for OSU's Wexner Medical Center, Landon is cooperating with the investigation and had denied knowing Epstein's years of abuse.
"I did not provide any clinical care for Jeffrey Epstein or any of his victims. I was a paid consultant for the New York Strategy Group regarding potential biotech investments from 2001 to 2005. I had no knowledge of any criminal activities; I find them reprehensible and I feel terrible for Epstein’s victims," he said.
The New York Strategy Group was Epstein's money management firm, records show.
Smith added: "[Landon] has stated he had no knowledge of any criminal activities and his consulting work did not involve any patient care. We continue to review the situation and have received no information to date that contradicts Dr. Landon’s statement."
Except for Landon and Victor, no other doctor has commented on being named or being associated to the convicted assaulter.
Christy Carlson Romano’s Cancer Test Result Shows Why Regular Screening Is Important

Credit: Instagram
American actress Christy Carlson Romano’s shocking announcement of a positive cancer screening test has reignited the debate on the rising early onset of cancers among women, as well as the importance of early screening.
Cancer is everywhere, said Romano, 41, in a tearful video on social media platform Instagram.
The former Disney star noted that she underwent cancer screenings, along with her husband, Brendan Rooney, as her family has a history of cancer.
“My husband’s came back completely negative,” she said, adding, “Mine did not come back negative. So basically, what that means is that I may have stageable cancer.”
The ‘Even Stevens’ star shared that she next aims to get a PET scan, which will help her gauge the stage of the cancer.
Early-onset Cancers Rising Among Women Under 50
While the news of Romano's positive cancer test has left fans shocked, it also highlights the fact that cancer is increasingly shifting its attack to women under 50.
A 2025 study by Duke Cancer Institute in the US revealed that for women younger than 50, the risk of developing cancer is 82 percent higher than that of men, up from 51 percent in 2022.
The 2025 annual report from the American Cancer Society (ACS) also showed that cancer rates in young and middle-aged women are rising past those of men in the same age group, but especially among women under age 50.
While breast cancer has emerged as the most common among women under 50, it is followed by thyroid cancer, melanoma, and skin cancers. Cervical cancer, ovarian cancer, and colorectal cancer are other names. Uterine cancer, also known as endometrial cancer, is also killing more women than ever.
Notable names include Princess of Wales Kate Middleton, who was 42 when she announced her cancer diagnosis two years back. American actress Olivia Munn reported a breast cancer diagnosis at the age of 43.
Similarly, Indian actress Sonali Bendre was 46 years old when diagnosed with high-grade metastatic cancer in July 2018. Others with young cancer onset include Hina Khan, Chhavi Mittal, Dipika Kakkar, and Tahira Kashyap. Young TV actresses Dolly Sohi and Priya Marathe reportedly succumbed to cancer.
According to studies, besides genetic factors, increased intake of highly processed foods, sugary beverages, lack of exercise, the surge in stress levels, exposure to air pollution, microplastics, and mindless intake of antibiotics, and even increased screen time are major contributors to the deadly disease.
Other possible risk factors include alcohol consumption, sleep deprivation, smoking, and obesity.
Why Is Early Screening Important?
Late diagnosis is a major driver of cancer deaths. On the contrary, early screening can help detect changes in the body's cells before cancer develops and spreads.
It can also help in cancers, which present no symptoms until the late stage.
It not only improves survival rates but also helps with less invasive and more effective treatments.
Regular screening measures include mammograms, Pap smears, colonoscopies, Low-dose CT Scans, and PSA tests, which reduce mortality by identifying cancer at early and manageable stages, preventing its progression and the risk of death.
© 2024 Bennett, Coleman & Company Limited

