- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Wastewater Surveillance In India: Can Sewage Help Detect Viruses Before Outbreaks?

Credits: Health and me
The Indian Council of Medical Research (ICMR) is embarking on one of its most ambitious public health projects yet—expanding wastewater surveillance from five cities to 50 within the next six months. The program will track 10 different viruses, including influenza strains and pathogens responsible for fever, diarrheal illnesses, encephalitis, and respiratory infections.
The goal is simple but powerful: to create an early warning system for outbreaks before they overwhelm hospitals. For India, a country with a vast and densely packed population, the ability to detect viral threats at the community level could be transformative. For the rest of the world, the project provides a case study of how wastewater-based epidemiology (WBE) can help governments prepare for and potentially prevent pandemics.
What Is Wastewater Surveillance?
Wastewater surveillance, also called Wastewater-Based Epidemiology (WBE), is the practice of analyzing sewage for fragments of viruses, bacteria, and other pathogens that people shed when they use toilets, sinks, or showers.
An infected person symptomatic or not will excrete viral particles through stool, urine, or even when washing. These particles end up in community sewage. When scientists collect and test these samples, they can detect infection trends in entire populations, sometimes weeks before clinical cases surface in hospitals.
Unlike hospital-based testing, which only captures people who seek medical care, wastewater surveillance gives a panoramic view of community health, including those who are asymptomatic, undiagnosed, or reluctant to get tested.
Why India Is Betting Big on Sewage Science?
Currently, India uses wastewater surveillance to monitor COVID-19 and polio. But with ICMR’s expansion plan, the program will track up to 10 viruses. Among them: avian influenza viruses and pathogens associated with acute encephalitis syndrome, diarrheal outbreaks, and respiratory infections.
This move isn’t just about academic curiosity. It’s about preparedness. India, like many other countries, is witnessing a rise in emerging and re-emerging pathogens fueled by rapid urbanization, climate shifts, increased human-animal interaction, and dense living conditions. With its massive population and vulnerable healthcare infrastructure, early detection is not optional, it’s essential.
The scaling up from five cities to 50 represents a tenfold leap in capacity, one that could significantly strengthen India’s ability to anticipate outbreaks and allocate resources before a crisis spirals.
How Wastewater Surveillance Process Works?
The science behind wastewater surveillance follows a clear workflow:
Pathogen Shedding – People infected with a virus shed particles into sewage through waste or while washing.
Collection – Samples are taken from untreated sewage at pumping stations or before treatment plants.
Laboratory Testing – Viral fragments (RNA/DNA) are extracted and tested using advanced molecular methods.
Data Analysis – Trends in viral load are mapped, typically providing a snapshot of community infections within 5–7 days.
Public Health Action – Authorities can respond with outbreak alerts, vaccination drives, and resource mobilization.
In short, wastewater turns into a real-time diagnostic tool—not for individuals, but for entire neighborhoods and cities.
Was COVID-19 First Detected Through Wastewater Surveillance?
If there’s one proof of concept for WBE, it’s the COVID-19 pandemic. In India, a study from Mumbai showed the SARS-CoV-2 virus was detectable in wastewater up to three weeks before clinical diagnoses surged. In Pune, scientists detected the XBB strain months ahead of physicians reporting the first confirmed cases.
Across the world, from Sydney to San Diego, cities leveraged wastewater as an important gauge of viral spread, enabling policymakers to coordinate with greater precision by timing restrictions, calibrating testing, or initiating vaccination campaigns.
This forecasting ability is precisely why India's growth is important. Picking up on early warning signs in sewage might be the difference between a localized outbreak and a national crisis.
How Does Wastewater Surveillance Addressing More Than Just Viruses?
Perhaps the most underestimated use for wastewater monitoring is to monitor antimicrobial resistance (AMR)—an invisible international threat that might render many antibiotics obsolete.
India already has an AMR surveillance program in place through a network of 60 hospitals tracking which medicines are effective against which infections.But this only captures patients who make it to hospitals. Wastewater surveillance can reveal resistance patterns at the community level, detecting resistant pathogens carried by people who never seek treatment.
Given projections that AMR could kill 10 million people annually worldwide by 2050, this kind of broad, real-world data is critical.
How Wastewater Surveillance Can Predict Viral Outbreaks?
India’s program is ambitious, but it’s part of a larger global shift. The U.S. Centers for Disease Control and Prevention (CDC) already uses wastewater to monitor COVID-19 and other pathogens. In Australia, Dr. Jiaying Li and her team at the University of Sydney developed wastewater methods to track not only viruses but also “forever chemicals” and illicit drug residues, showing the technique’s versatility.
These international examples highlight why public health experts call WBE the “stethoscope of cities.” It listens to what individuals may not yet know about their health and helps leaders act before hospitals get overwhelmed.
For India, timing is everything. The country has weathered devastating outbreaks—from the 2009 H1N1 flu to COVID-19’s Delta surge—and its public health infrastructure is still catching up. Traditional syndromic surveillance systems (tracking patients with fever, cough, or diarrhea) are already in place but rely on people showing up at hospitals.
Wastewater surveillance changes that equation. It brings data from households, schools, workplaces, and entire communities—even those who never set foot in a clinic. That means potential hot spots can be identified and interventions rolled out before the first wave of hospitalizations.
The surveillance will be carried out through ICMR’s national network of Viral Research and Diagnostic Laboratories, which already tests about 1,500 patient samples a week for respiratory illnesses. Adding wastewater to the mix gives India a more layered, resilient system of outbreak detection.
If successful, the program could eventually scale nationwide and serve as a model for other low- and middle-income countries. Integrating wastewater surveillance with India’s Ayushman Bharat Digital Mission (a massive health data initiative) could turn real-time sewage signals into actionable alerts for policymakers and communities alike.
Wastewater may not be glamorous, but it might just be one of the most powerful public health tools of the 21st century. By expanding its surveillance network to 50 cities, India is not just strengthening its defenses against outbreaks it’s offering the world a glimpse of how proactive, community-level monitoring could rewrite the rules of epidemic preparedness.
Chikungunya Vaccine License Suspended In U.S. By FDA

Credits: Canva
The U.S. Food and Drug Administration (FDA) has suspended the biologics license for Ixchiq, a live-attenuated chikungunya vaccine, following reports of serious adverse reactions in older adults. The side effects closely mirrored symptoms of chikungunya virus itself, raising concerns about patient safety and the vaccine’s overall clinical benefit.
Chikungunya is a mosquito-borne viral infection that causes high fever, rash, headaches, nausea, fatigue, and severe joint and muscle pain. The illness is notorious for its long-lasting impact, with some patients experiencing disabling joint pain for months or even years.
Global rise in chikungunya cases
The FDA decision comes at a time when chikungunya cases are surging worldwide. In recent months, outbreaks have been reported across South America, Asia, and Africa. The World Health Organization (WHO) has noted significant increases in Brazil, Paraguay, and India, with localized outbreaks also being reported in parts of Southeast Asia.
In the United States, sporadic travel-related cases have been documented, though no major outbreak has yet occurred. Concern is growing that climate change and global travel could expand the reach of chikungunya-carrying mosquitoes to new regions.
U.S. issues travel advisory for China amid outbreaks
Adding to global health concerns, the U.S. recently issued a travel advisory for Americans visiting China after reports of chikungunya cases in several provinces. Health officials have urged travelers to take preventive measures such as using mosquito repellents, wearing long-sleeved clothing, and staying in well-screened accommodations. The advisory highlights the urgency of preventive strategies as global cases rise.
Read: US Weighs China Travel Warning As Chikungunya Cases Near 5,000: Report
Safety issues with Ixchiq vaccine
Ixchiq, developed by French biotech company Valneva, received accelerated FDA approval in November 2023. It was intended to protect adults aged 18 and older at increased risk of exposure to the virus. However, post-approval monitoring has revealed troubling safety signals.
According to the FDA’s Vaccine Adverse Event Reporting System (VAERS):
- One confirmed case of encephalitis-related death has been linked to the vaccine.
- More than 20 serious adverse events resembling chikungunya illness have been reported.
- 21 hospitalizations and three total deaths have been associated with vaccination.
The FDA’s Center for Biologics Evaluation and Research concluded that Ixchiq’s clinical benefits remain unproven and that its risks outweigh potential advantages for most individuals. Regulators determined that continued use in the U.S. could jeopardize public health.
Manufacturer defends vaccine but halts U.S. distribution
Valneva, the vaccine’s manufacturer, has defended its product, stating that the reported side effects align with known risks identified during clinical trials. The company emphasized that older adults had already been flagged for potential complications in prescribing information.
“As we determine potential next steps, and as the clear threat of chikungunya continues to escalate globally, Valneva remains fully committed to maintaining access to our vaccine as a global health tool for addressing and preventing outbreaks of this devastating illness,” Valneva CEO Thomas Lingelbach said in a statement.
The company has confirmed that while Ixchiq will no longer be shipped or sold in the U.S., it will continue to be made available in other countries where it is licensed. Valneva also reiterated its commitment to accelerating vaccine access in low- and middle-income nations where chikungunya remains endemic.
Trump And RFK Jr. Could Pull COVID Vaccine Off U.S. Market ‘Within Months’

Credits: AFP and Canva
US President Donald Trump and Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. are allegedly preparing to phase out COVID-19 vaccines “within months,” according to a close associate of Kennedy. The move, if true, would mark a dramatic reversal for Trump, who once hailed the vaccine’s rapid development during the pandemic as a “monumental achievement.”
An HHS spokesperson told Newsweek that the agency does not comment on potential policy decisions. The White House has dismissed the claims as “baseless speculation.”
Also Read: Chikungunya Vaccine License Suspended In U.S. By FDA
Trump’s Shift From Championing to Questioning Vaccines
Back in December 2020, at the height of the pandemic, Trump described the rollout of vaccines under Operation Warp Speed as a “historic success.” He praised scientists, pharmaceutical companies, and government agencies for developing and distributing the shots in record time.
But the political landscape around vaccines shifted in the years that followed. Segments of Trump’s base began raising doubts about vaccine safety and effectiveness, fueling conspiracy theories and distrust of public health guidance.
RFK Jr.’s Anti-mRNA Vaccine Stance
Kennedy, who has long been criticized for spreading vaccine skepticism, insists he is not “anti-vaccine.” Earlier this month, he announced HHS would pull $500 million in federal funding from 22 mRNA vaccine development projects, arguing that data shows they “fail to protect effectively against upper respiratory infections like COVID and flu.”
Critics, however, say Kennedy’s statements echo long-debunked claims and risk undermining public trust in science. His leadership at HHS has already sparked petitions demanding his removal, with one Change.org campaign backed by more than 45,000 signatories from physicians and medical students.
Associate Claims Vaccine Ban Coming “Within Months”
Dr. Aseem Malhotra, a British cardiologist and vocal vaccine skeptic, told The Daily Beast that Kennedy’s position is supported by “influential” members of Trump’s family. Malhotra, an adviser to the lobby group Make America Healthy Again Action, claimed those close to Kennedy believe it is “incomprehensible” that the vaccine remains available.
He suggested the vaccine could be withdrawn in stages, pending further review of alleged “vaccine injuries,” or removed entirely in a single sweeping decision. Such a move, he admitted, could cause “fear of chaos” and carry major legal consequences.
The White House quickly pushed back. “The Administration is relying on Gold Standard Science and is committed to radical transparency,” spokesperson Kush Desai said. “Unless announced by the Administration, any discussion about HHS policy should be dismissed as speculation.”
Contested Evidence and Scientific Pushback
Malhotra cited a 2022 paper in the journal Vaccine, which claimed recipients of Pfizer and Moderna shots had a 16% higher risk of serious adverse events compared to placebo groups. However, mainstream medical experts have dismissed the study as flawed and misleading, stressing that the overwhelming consensus remains that COVID-19 vaccines are safe and effective in preventing severe illness and death.
Public health specialists warn that even rumors of a vaccine ban could discourage people from getting recommended seasonal shots against flu, RSV, and COVID, increasing the risk of severe outbreaks. “Even just rumors are likely to reduce uptake,” said Dr. Jessica Holzer of the University of New Haven, as reported in Newsweek.
Confusion Among the Public
Experts fear that mixed signals from the Trump administration could deepen public uncertainty. “Scientific consensus is clear: vaccination benefits outweigh the risks for most people,” said Dr. Amy Bucher, chief behavioral officer at Lirio to Newsweek. She warned that wavering government positions risk eroding trust, fueling polarization, and emboldening conspiracy theories.
According to Bucher, emphasizing personal choice is key to easing resistance. “If people feel it’s their decision, they’re more open to information. But if vaccines are taken off the market entirely, it could spark stronger backlash and worsen public health outcomes.”
What’s Next
For now, the administration has not formally announced any policy changes. Still, Kennedy’s influence within HHS and Trump’s evolving rhetoric suggest the vaccine debate will remain a flashpoint in U.S. politics.
A petition to remove Kennedy from office continues to gather momentum, underscoring the fierce divide over vaccine policy. Whether speculation hardens into official action could shape not only America’s public health trajectory but also Trump’s political legacy.
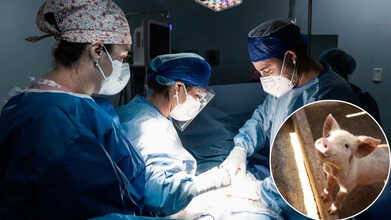
Surgeons Transplant First-Ever Gene-Edited Pig Lung Into A Brain-Dead Man
Credits: Health and me
In a breakthrough that will possibly redefine the future of organ transplantation, a team of surgeons in China have successfully performed a transplant using a genetically modified pig lung into the chest of a 39-year-old brain-dead man. The lung functioned for nine days and opened up unprecedented insights into xenotransplantation, the practice of transplanting animal organs into humans. Although the experiment was short-lived, it has ignited hope to amass the millions left waiting each year for life-saving transplants.
For decades, the worldwide shortage of donor organs has stranded millions waiting for a life-giving transplant that might never occur. In the United States alone, over 103,000 individuals were waiting in line for an organ transplant in 2023, but fewer than half received one. Each day, approximately 13 patients die while waiting. And now, an experimental procedure from China is testing the limits of science: for the first time, surgeons have implanted a genetically engineered pig lung into a human.
Also Read: Chikungunya Vaccine License Suspended In U.S. By FDA
The recipient, a 39-year-old male who was declared brain-dead following a hemorrhage, survived for nine days with the transplanted lung before the experiment was terminated at the request of his family. Although the organ did not survive long-term, the procedure represents a dramatic advance in xenotransplantation, the utilization of animal organs in humans.
On May 15, 2024, a surgical team at the First Affiliated Hospital of Guangzhou Medical University performed the historic surgery. The donor organ was taken from a genetically modified miniature pig with six CRISPR edits intended to render it compatible with human biology. The edits silenced three pig genes that induce immune rejection and introduced three human genes that control inflammation and immune response.
The lung was carefully transplanted into the man's chest; the other lung was left intact to support function. Over the following hours, the medical team watched for signs of "hyperacute rejection," the immediate and catastrophic immune response that usually destroys foreign organs within minutes. To their surprise, this did not occur.
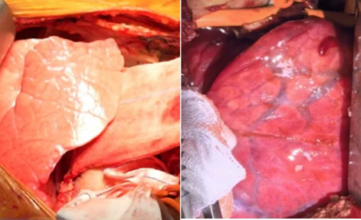
But soon difficulties arose. By the 24-hour mark, the new lung began to develop inflammation and fluid retention. By day three, there was antibody-mediated rejection. Although there were initial indications of stabilization, the transplant lung was unable to combat the overwhelming immune response. On day nine, in accordance with family desires, physicians discontinued the experiment.
Why Lungs Are Harder to Transplant?
While kidneys and hearts have challenges to overcome in transplantation, such as size matches, lungs have their own set of challenges. Their intricate structure, suited for oxygen and carbon dioxide exchange, predisposes them to injury and inflammation. In addition, lungs are the only transplanted organ completely exposed to the external environment — each breath introduces bacteria, viruses, pollen, and toxins that the immune system needs to screen out.
Achieving the balance between suppressing the immune system sufficiently to avoid rejection but not so much that the patient is lethally susceptible to infection has been one of medicine's most formidable challenges. Indeed, repeated success with human-to-human lung transplants was only made possible in the 1980s, two decades after kidney and heart surgery had been successfully accomplished. Introducing cross-species transplantation into the equation yet again increases the science's complexity.
The Global Organ Shortage Crisis
The World Health Organization has projected that only 10% of the world's demand for organ transplants is now being filled. In the United States, the supply-demand gap increases yearly even with attempts to raise donor registration levels. With developments in organ preservation and sharing networks, still thousands of patients never receive the opportunity for an operation.
This is where xenotransplantation may revolutionize things. Pigs have emerged as the animal donor of choice due to their organ size, physiological proximity to humans, and ease of genetic modification. Pig heart valves have been successfully implanted in humans for over 30 years. Genetically altered pig kidneys and hearts have been transplanted into human recipients more recently with varied degrees of short-term success.
Historical Timeline of Xenotransplantation Milestones
Over the last few years, there have been a proliferation of milestone procedures that have registered global attention:
- In 2022, American surgeons implanted a genetically altered pig heart into a living patient, who lived for two months.
- Last January 2024, a Massachusetts man received a genetically altered pig kidney and remains alive with the organ functioning.
- Chinese scientists tried to transplant a pig liver into a brain-dead patient, results being limited.
The pig lung transplant represents a new frontier, pushing the boundaries of xenotransplantation research. Every effort gives valuable information regarding how animal organs respond in humans and what the largest hurdles are.
What Makes This Pig Lung Different?
The pig lung utilized in the Guangzhou experiment was obtained from a Bama miniature pig kept in a pathogen-free facility by Clonorgan Biotechnology based in Chengdu, China. This provided the assurance that the animal was not exposed to infections that would otherwise be passed on to the human recipients.
It is the CRISPR alterations that rendered this experiment possible. By deleting pig genes for sugars identified as "foreign" by the human immune system and adding human immune-regulating proteins, researchers sought to "humanize" the lung. Although the rejection reaction still happened, the failure of hyperacute rejection — long the most immediate hurdle — to occur indicates that gene editing is closing the gap.
What Are The Medical and Ethical Challenges Beyond This Medical Miracle?
Despite this milestone, experts caution that pig lungs won’t be saving lives in hospitals anytime soon. The risks of infection, rejection, and long-term complications remain significant. In addition, the ethical considerations of using animal organs, particularly genetically modified ones, are far from resolved. Critics argue that resources might be better invested in advancing bioengineered or lab-grown organs rather than relying on animals.
But to patients presented with the stark reality of organ deficiencies, the prospect of a limitless alternative source is difficult to resist. Families who agree to such experimental surgeries do so in the belief that their contribution will help pave the way for a future where others will not have to wait in vain.
The results of the Guangzhou team, reported in Nature Medicine, highlight both the potential and dangers of xenotransplantation. Though rejection is still the biggest obstacle, the advancements within a few years are remarkable. What was previously considered science fiction is slowly becoming a reality, though through industrious trial and error.
For the time being, lung xenotransplantation is an experimental frontier and not a clinical option but as genetic engineering progresses and our understanding of immune regulation becomes more profound, it may one day be able to assist in revolutionizing transplantation medicine. If researchers can one day bypass the immune system's hurdles, the 103,000 individuals already on the US transplant waiting list and hundreds of thousands worldwide may at last have new hope.
© 2024 Bennett, Coleman & Company Limited

