- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
We All Could Carry The Epstein-Barr Virus That Triggers Lupus, According To Stanford Scientists
Credits: Canva
A new study published in the Science Translational Medicine has revealed a connection between the virus called the pstein-Barr virus (EBV), a pathogen silently carried by almost 95% of adults with systemic lupus rythematosus, better known as lupus.
This Stanford-led research shows that EBV could highjack a tiny subset of immune cells and push the body into a full-blown autoimmune attack. It uses ultraprecise sequencing technique to do so, which scientists have finally been able to tract down in the infected immune cells and have been able to uncover how just a handful of them could lead to widespread inflammation. As per the researchers, the mechanism could explained virtually all lupus cases.
Why Lupus Has Been So Hard to Explain
Lupus impact is severe as it affects hundred thousand Americans, and has an estimate of 5 million people being affected with this worldwide. It is a chronic autoimmune disorder in which the immune system mistakenly attacks the nuclei of the body's own cells, and leads to damage across organs. These organs include anywhere from skin, joints, to heart, kidneys, and even the nervous system.
A reason that continues to remain mysterious is why 90% of lupus patients are women. While medications can slow the disease and help most people live normal lives, the condition can be life-threatening for about 5% of patients. Despite decades of research, scientists have struggled to pinpoint a single trigger. EBV has always been a suspect, but until now, no one had the tools to prove it.
You May Have The Virus That Triggers Lupus
Despite the devastating truth about lupus, almost everyone has EBV, the virus that could trigger it. It is so common that even one researcher joked that the only way to avoid is be living "in a bubble".
How does it spread? EBV usually spreads through saliva, by sharing utensils as kids, or kissing. It can cause mononucleosis, the classic “kissing disease,” which often leaves people with lingering fatigue.
What makes this tricky is that once EBV infects you, it never fully leaves. It is like herpes or chickenpox viruses, which could tuck its genetic material into the nuclei of infected cells and lies dormant, sometimes, throughout life. Among its favorite hiding spots are B cells, powerful immune cells responsible for producing antibodies and activating other parts of the immune system.
Typically, only a tiny fraction of someone’s B cells carry EBV, making them nearly impossible to detect with older scientific tools.
What Does This Breakthrough May Mean For Finding These EBV Inside Rogue B Cells?
Using their new high-precision sequencing method, the Stanford team discovered that in a healthy person, fewer than 1 in 10,000 B cells carry EBV. But in patients with lupus, the ratio jumps dramatically to 1 in 400—a 25-fold increase.
This matters because EBV-infected B cells occasionally produce an important viral protein called EBNA2. EBNA2 behaves like a genetic on-switch. It activates dormant human genes, many of which are involved in inflammation. Once switched on, these B cells turn into aggressive, hyper-inflammatory antigen-presenting cells. They, in turn, rally other immune cells, especially helper T cells, to join the attack.
Those T cells then recruit hundreds of other B cells that mistakenly target the nuclei of healthy cells. The result? A runaway chain reaction that leads to the formation of antinuclear antibodies, the hallmark of lupus.
Critically, most of the B cells joining the attack are not EBV-infected. But once the inflammatory cascade begins, EBV need not be present in every cell. It only needs a few instigators.
Do Everyone With EBV Get Lupus?
While nearly all of us has EBV, only some people develop autoimmune diseases. This could be because certain EBV strains may be more likely to convert B cells into the 'driver cells' that would kick-start autoimmunity. Genetics too plays an important role in how someone's immune system responds to these infected cells.
This new finding therefore just does not offers a broader understanding of lupus, but also opens doors for treatment strategies. Many biotech companies are already developing EBV vaccines, though these would need to be given in early infancy because vaccines cannot clear an existing infection.
There’s also interest in aggressive therapies like ultradeep B-cell depletion, which aims to wipe out all circulating B cells and allow new, EBV-free cells to grow back.
Researchers say the same EBV-triggered mechanism may play a role in other autoimmune conditions, including multiple sclerosis, rheumatoid arthritis, and Crohn’s disease.
Erythritol Sweetener Could Be Linked To Stroke Risk, Finds Study

Credits: Canva
Erythritol sweetener, commonly found in most of the food we consume, whether it is a protein bar or energy drink could be linked to stroke risk. While it is considered as a safer alternative to sugar as a natural sweetener, a study from the University of Colorado suggests it could damage cells in the blood-brain barrier.
The blood-brain barrier is brain's security system that keeps the harmful substance off the limits, while letting in nutrients. Research also suggests that it would lead to serious consequences for heart health and stroke risk.
Erythritol Sweetener Risk: What Did The Study Find?
In the latest study, researchers exposed cells that form the blood–brain barrier to erythritol levels typically seen after consuming a soft drink sweetened with the compound. What followed was a cascade of cellular damage that could leave the brain more vulnerable to blood clots, one of the leading causes of stroke.
The researchers found that erythritol triggered intense oxidative stress, overwhelming cells with unstable molecules known as free radicals. At the same time, it weakened the body’s natural antioxidant defences. This double hit impaired normal cell function and, in some cases, led to cell death.
Damage to blood–brain barrier cells is particularly concerning because this barrier plays a crucial role in protecting the brain from harmful substances circulating in the bloodstream. When its integrity is compromised, the risk of neurological injury rises sharply.
Erythritol Sweetener Risk: How It Disrupts Blood Flow Control
Even more troubling was erythritol’s effect on how blood vessels regulate blood flow. Healthy blood vessels constantly adjust their width—expanding when organs need more oxygen and nutrients, and narrowing when demand is lower.
This process depends on a delicate balance between two molecules: nitric oxide, which relaxes blood vessels, and endothelin-1, which causes them to constrict. The study found that erythritol disrupted this balance by reducing nitric oxide production while increasing endothelin-1 levels.
The result is blood vessels that stay constricted longer than they should, potentially restricting blood flow to the brain. This kind of dysfunction is a known warning sign for ischaemic stroke, the most common form of stroke caused by blocked blood vessels.
Erythritol Sweetener Risk: How It Interferes With Body's Clot Defense
The most alarming finding in the study was how body's natural protect against blood clot is disturbed. Under normal circumstances, cells release a substance called tissue plasminogen activator, which is described as a natural 'clot buster', which helps dissolve clots before they become dangerous. However, erythritol could interfere with this protective mechanism and allow clots to persist and cause damage.
Several have shown that people with higher blood levels of erythritol face significantly increased risks of cardiovascular events. In one major study, individuals with the highest erythritol levels were nearly twice as likely to suffer a heart attack or stroke.
However, researchers caution that the experiments were conducted on isolated cells rather than full blood vessels. More advanced models that better replicate human physiology will be needed to confirm the findings.
Erythritol occupies a unique space in the sweetener world. Classified as a sugar alcohol rather than an artificial sweetener, it escaped recent World Health Organization guidance discouraging artificial sweeteners for weight control. Its sugar-like taste has also made it a favorite in “keto-friendly” and sugar-free foods.
FDA Refuses To Review Moderna's Flu Vaccine Application
Credits: Canva
FDA refuses to review Moderna's flu vaccine: The U.S. Food and Drug Administration (FDA) has declined to begin reviewing Moderna’s application for its experimental flu vaccine. The company made the announcement on Tuesday. The decision marks another signal of stricter vaccine oversight under the Trump administration and has already rattled investor confidence, with Moderna’s stock falling nearly 7% in after-hours trading.
Read: CDC Vaccine Schedule: Coverage Falls From 17 to 11 Diseases For Children
Moderna said the FDA’s refusal came as a surprise and contradicted feedback the company had received earlier, before it submitted the application and launched phase three trials for the vaccine, known as mRNA-1010. The company has now requested a meeting with the agency to better understand what it described as an unclear “path forward.”
FDA Refuses: Objections Raised Over Trial Design
According to Moderna, the FDA did not flag any safety or efficacy concerns with the vaccine itself. Instead, the agency objected to the design of the clinical trial—despite having previously signed off on it. Moderna added that the setback would not affect its financial guidance for 2026.
The experimental flu shot had shown encouraging results in phase three trials last year, successfully meeting all primary trial endpoints. At the time, Moderna positioned the stand-alone flu vaccine as a critical step toward developing a combined influenza and COVID-19 vaccine, a key long-term goal for the company.
FDA Refuses: Shifting U.S. Vaccine Policy Landscape
The decision comes amid sweeping changes to U.S. immunisation policy over the past year under Health and Human Services Secretary Robert F. Kennedy Jr., who has long expressed skepticism toward vaccines. Moderna on Tuesday pointed to the FDA’s top vaccine regulator, Vinay Prasad, who returned to the agency in August after being removed earlier.
Prasad currently heads the FDA’s Center for Biologics Evaluation and Research (CBER) and has publicly argued for tighter regulatory standards for vaccines. He has also drawn controversy for comments linking child deaths to COVID-19 vaccines.
FDA Refuses: FDA Letter Cites Comparator Concerns
In a letter dated February 3 and signed by Prasad, the FDA stated that its refusal to review Moderna’s application was solely due to concerns about the trial’s design. Specifically, the agency objected to Moderna’s choice of comparator, arguing that comparing the experimental shot to a standard, approved flu vaccine did not represent the “best available standard of care.”
As a result, the FDA concluded that the study did not qualify as an “adequate and well-controlled” trial under its regulatory definition.
Moderna has strongly disputed this interpretation, arguing that FDA rules do not require companies to use the most advanced or highest-dose vaccine as a comparator in clinical trials.
FDA Refuses: Moderna Pushes Back, Eyes 2026–27 Timeline
In a statement, Moderna CEO Stéphane Bancel said the decision undermines innovation and fails to advance shared public health goals. He emphasized that the trial design had been discussed and agreed upon with CBER before the study began.
Moderna now expects the earliest possible approval for its flu shot to come in late 2026 or 2027, pending regulatory reviews across the U.S., Europe, Canada, and Australia.
The FDA declined to comment, stating it does not discuss regulatory communications with individual companies, reported CNBC.
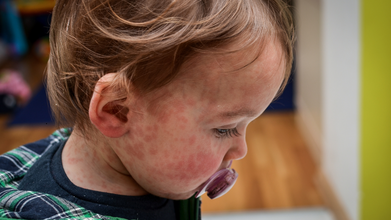
Measles Cases Cross 14,000 in Mongolia, Mostly Among Partially Vaccinated Children
Credits: Canva
Mongolia is witnessing a sharp rise in measles infections, with the total number of confirmed cases reaching 14,123, according to the country’s National Centre for Communicable Diseases (NCCD). Health officials say the outbreak is largely affecting school-age children, many of whom had received only one dose of the measles vaccine instead of the recommended two.
In a public advisory, the NCCD urged parents to ensure their children complete the full vaccination schedule, warning that partial immunization leaves children vulnerable to a potentially severe and highly contagious disease.
Why Children Are Most Affected
Health authorities noted that the majority of new infections were recorded among children who had not received their second measles shot. While a single dose offers some protection, it is not sufficient to prevent outbreaks, especially in school settings where close contact accelerates transmission.
The NCCD stressed that completing the two-dose regimen significantly strengthens immunity and reduces the risk of community-wide spread.
One of the World’s Most Contagious Viruses
Measles is considered one of the most infectious diseases known to humans. It spreads through direct contact with infected nasal or throat secretions, such as coughing or sneezing, and through airborne transmission in enclosed spaces.
What makes measles particularly dangerous is its ability to remain active in the air or on surfaces for up to two hours after an infected person has left the area. According to health experts, a single measles patient can infect up to 18 other people, making rapid containment extremely challenging once outbreaks begin.
Why Vaccination Still Matters
Vaccination remains the most effective way to prevent measles infection and limit its spread. The measles vaccine is safe, cost-effective and helps the immune system recognize and fight the virus before serious illness develops.
Before the measles vaccine was introduced in 1963 and widely adopted, large-scale outbreaks occurred every two to three years worldwide, causing an estimated 2.6 million deaths annually. Despite medical advances, measles continues to claim lives when vaccination coverage declines.
In 2023 alone, an estimated 107,500 people died from measles, most of them children under the age of five, highlighting the consequences of gaps in immunization programmes.
Recognising the Symptoms Early
Symptoms of measles typically appear 10 to 14 days after exposure. Early signs often resemble a common viral illness and can last up to a week. These include a runny nose, persistent cough, fever, red and watery eyes, and tiny white spots inside the mouth known as Koplik spots.
The most recognizable symptom—a red, blotchy rash—usually appears 7 to 18 days after exposure. It starts on the face and upper neck before spreading downward to the torso, arms, legs, hands and feet over several days. The rash generally lasts five to six days before fading.
A Renewed Call for Prevention
Health officials in Mongolia emphasize that measles outbreaks are preventable. They urge parents, caregivers and schools to prioritize full vaccination and seek medical advice at the first sign of symptoms.
With measles capable of spreading rapidly through communities, authorities warn that completing both vaccine doses is not optional but essential to protecting children and preventing future outbreaks.
© 2024 Bennett, Coleman & Company Limited

