- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
What Is 'Make America Healthy Again' All About?

Credits: Instagram: Robert F Kennedy Jr. Casey Means, and Alex Clark
President-elect Donald Trump coined 'MAGA', the Make America Great Again movement. It is a political movement which was used heavily during the presidential campaigns in 2016 and 2024. The latest development is its conservative sub-brand 'MAHA', Make America Healthy Again, with many MAHA influencers jumping right into the campaign, re-imagining the food policies and warning Americans on what they should or should not consume.
The forefront of MAHA is Robert F Kenny Jr. (RFK Jr.), who was recently nominated by Trump as the US Health Secretary, the position that awaits Senate approval. This battle incorporates RFK Jr.'s struggle against corporate agriculture, pharmaceutical companies and medical establishments.
Background
The externalised cost of food-related, noncommunicable diseases for Americans is over $1.3 trillion per year. This is greater than the value of all groceries sold annually. The food industry is also reaping high profits dealing with ultra-processed foods, many of which are banned in many other countries.
Robyn O'Brien, Chief Operating Officer at Montcalm says that 1 in 2 American men are expected to get cancer in their lifetimes, while 1 in 3 American women can expect the same. 1 in 3 American children has four As, which stands for allergies, autism, ADHD, and asthma, along with cancer as the leading cause of death in American children.
In this backdrop, it is JFK Jr.'s MAHA who wants to "dismantle the corporate stranglehold on [read the] our government agencies that has led to widespread chronic disease, environmental degradation, and rampant public distrust...MAHA seeks to drive a transformative agenda. This includes prioritizing regenerative agriculture, preserving natural habitats, and eliminating toxins from our food, water, and air… to combat the chronic disease epidemic, which includes addressing the root causes such as poor diet, environmental toxins, and inadequate healthcare... and dismantling the corporate takeover of government agencies that are supposed to protect public health and the environment.”
How did MAHA begin?
Calley Means and Dr Casey Means, a brother-sister duo built a wellness empire by questioning some of the traditional medical expertise and vaccine mandates. Their ideas also spoke to Trump's MAHA movement.
Calley who is a former food-industry lobbyist and Casey, a Stanford-educated surgeon, have pushed for a revamp of the American food and health system. This is driven by a deep distrust of the pharmaceutical and food industries and medical theories. Drawing on this thought, JFK Jr. also mentioned both siblings as people he would recommend to Trump for "prominent roles" in his administration.
The siblings have criticised the approach that the US medical systems are taking. They say, that instead of going for a holistic look at the body's needs through nutrition, exercise and health, the current medical system is relying on medications.
Calley also called the Covid-19 vaccine mandates a "war crime", and that parents should rely on the "divine gifts of intuition and heart intelligence" rather than "blindly trusting the science".
ALSO READ: Who is Robert F. Kennedy Jr., the Controversial Nominee for U.S. Health Secretary?
MAHA Influencers
There are MAHA influencers other than the podcaster siblings and JFK Jr. himself. Enters Alex Clark. "Cool girl, loves health and wellness, and happens to be conservative", the 31-year-old introduces herself.
She is able to tap into those who are not politically aligned, but are worried about their kids' health. In a podcast called Realfoodology, she and Courtney Swan, a nutritionist look for chickpeas, which might "slowly poison you", as Clark believes.
These chickpeas contain glyphosate, which as per the Environmental Protection Agency has "no risks of concern to human health". The Food and Drug Administration (FDA) noted that one international organisation said that it may be a carcinogen, but no other organisation has found or said anything. But for Clark, it does not matter because she has no faith in the "three-letter-agencies".
She in her podcast Culture Apothecary promoted MAHA, discussing various issues on health and wellness, with the top discussion on motherhood as a virtuous role, daycare as dangerous and feminism as insidious.
MAHA is a big no on hormonal birth control and she too is a promoter of the idea that the women's liberation movement has done more harm than good. Aligning with Republican's anti-abortion views, this MAHA influencer is a firm believer that abortion is "never medically necessary to save the life of the mother," even if the experts disagree.
What Else MAHA Wants?
MAHA is against regular mammogram testing because it exposes women to radiation and disrupts their body's function. As per the National Cancer Institute, the benefits of undergoing regular screenings exceed the risk of skipping them.
Another thing many MAHA believers hold deeply close to is that "my body, my choice" is just an illusion created by the state. In reality, the body is controlled by medicines, which leads to autism in children. The popular belief is that the food Americans eat, controlled by the corporation is what makes them sick, and they seek medical help, which controls their brain. It is a vicious cycle and MAHA wants to break free.
MAHA does not want these government agencies to co-parent their kids. They are clear that they want a "divorce", as Clark says.
With JFK Jr. being nominated as the US Health Secretary, who could control the health agencies, including the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and many more, MAHA could be a dream come true in "divorcing" the parents with the government.
Canada Top Court Says COVID Travel Restrictions Broke Mobility Rights Within The 'Reasonable Limits'

Credits: iStock and Canva
Travel Restrictions To Canada: The top court of the land, that is the Supreme Court of Canada ruled that travel restrictions were violative of citizen's right, however, are reasonable during "grave emergency" like pandemic.
Travel Restrictions To Canada: What Happened That Led To Supreme Court Ruling?
During May 2020, in early days of the COVID-19 pandemic, Kimberley Taylor, who was living in Halifax, got the news of her mother Eileen's passing away at the age of 75 of natural causes at St John's, as reported by The Global Mail. Kimberley wanted to attend a small memorial service however was unable to go there due to the Newfoundland government's rejection of her initial request.
The memorial service and the burial was attended by her father, sister and niece. "I was denied the ability to join my family to grieve my mother," she said, as reported.
This is when she along with the Canadian Civil Liberties Association launched a legal challenge. They noted that strict travel restrictions were in violation of the mobility rights within Canada as guaranteed by Section 6 of the Charter of Rights and Freedoms.
Last week, on Friday, a nine judge bench in the Supreme Court of Canada provided an expansive view of the Canadians' Charter mobility rights.
Travel Restrictions To Canada: What Did The Canadian Supreme Court Say?
The top court said that while Kimberley's rights were in fact violated by Newfoundland's pandemic rules, these were within the reasonable limits. The court deemed the pandemic travels rules to be in bounds within the reasonable limits on rights and freedoms in Section 1 of the Charter.
Jessica Kuredjian, a lawyer at Cassels in Toronto, as reported by The Global Mail, said, "This is a great ruling, and an important one. It is very human case. It is a great example of where Charter rights impact the real rights of everyday citizens."
The ruling will serve as a precedent for the ambit of government actions and restrictions during potential health emergencies in the future.
In a majority ruling authored by Justices Andromache Karakatsanis and Sheilah Martin, the court noted that early pandemic deaths and scientific uncertainty created an “extraordinarily difficult situation,” requiring swift decisions to protect public health and prevent further loss of life.
The judgment marks the latest major court effort to define the balance between individual freedoms and government authority stemming from pandemic-era actions. Just last month, the Federal Court of Appeal found the federal government’s 2022 use of the Emergencies Act to curb large protests was not legally justified.
Friday’s ruling also adds to the legal interpretation of the 1982 Charter of Rights. While many Charter provisions have been heavily litigated over the years, Section 6 — mobility rights — has rarely been tested in court.
“There really was a dearth of jurisprudence on the topic,” said Anaïs Bussières McNicoll, director of the Civil Liberties Association’s fundamental-freedoms program.
The pandemic travel-restriction case effectively marks the Supreme Court’s first detailed examination of Section 6 as it applies to Canadians’ general freedom of movement within the country.
In their majority opinion, five judges stressed that Charter rights must receive “generous protection.” On mobility rights, they traced the concept back nearly a millennium, linking it to common-law traditions from the 1200s and even earlier “ancient customs.”
North London Measles Outbreak: 34 Cases Confirmed In Unvaccinated Children From Enfield
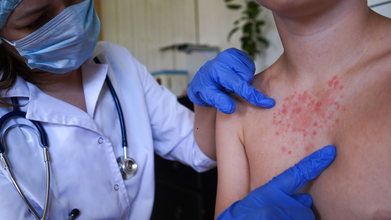
Credits: iStock
North London Measles Outbreak: 34 children have been infected by a "fast spreading" measles outbreak in several north London schools, confirmed health officials. The cases were first confirmed from Enfield in laboratory tests in January, as is reported by the UK Health Security Agency or the UKHSA.
A local GP, as reported by the BBC said that one in fiver children who contracted the illness had been admitted to hospital. The doctor also said that these children "had not been fully immunized".
Families are now asked to ensure that their children are up to date with their immunizations and vaccinations against this highly contagious disease. Measles could cause serious health complications.
North London Measles Outbreak: Who Can Get The Vaccines?
Measles vaccinations for children are available at the school, however, if they missed it, they can also get it at a number of catch-up clinics around the UK. The vaccinations are for free.
Enfield's NHS Ordnance Unity Centre For Health on its website noted that there is a "fast spreading measles outbreak in several schools" across the borough. The infections were confirmed in "at least" seven schools in Enfield, which means there could be more. Some reports also came from neighboring Haringey.
Enfield Councillor Alev Cazimoglu said that current outbreak had "mainly affected children and some have required additional care with a short stay in hospital". She also said, "Vaccination is the most effective way to protect yourself and your family. We urge everyone who is not fully vaccinated to act now."
The 34 cases of Enfield represent over a third of the 96 total cases which were confirmed in England in the first month of this year as per the UKHSA data.
As per the Enfield Council, it is working closely with UKHSA, the NHS and local partners to limit any further spread.
Read: UK Loses Measles Elimination Status: Why Is This Disease Making A Comeback?
North London Measles Outbreak: Who Are At Most Risk?
As per a UKHSA medical practitioner, Dr Vanessa Saliba, as also reported by the BBC, the "big" outbreak is "mostly affecting unvaccinated children under 10 in schools and nurseries". She also added, "Measles is a nasty illness for any child, but for some it can lead to long term complications and tragically death, but is so easily preventable with two doses of the MMRV [measles, mumps, rubella, chickenpox] vaccine."
Dr Saliba also suggested children to catch-up with their vaccine schedule in case they have missed it and also urged those travelling abroad over the Easter holidays to check their vaccination status.
North London Measles Outbreak: What Is It And What Are The Symptoms?
Measles is a highly contagious disease. It spreads by coughs or sneezes or by touching things that someone with measles has coughed or sneezed on.
Measles, also known as rubeola, is an extremely contagious viral illness that typically causes high fever, cough, runny nose, red and watery eyes, and a characteristic rash that begins on the face and spreads downward across the body. It spreads through respiratory droplets and can lead to severe and sometimes fatal complications, including pneumonia and inflammation of the brain known as encephalitis.
Symptoms include high fever, sore or red and watery eyes, coughing, sneezing, and small white spots in the mouth.
New Monkeypox Variant Emerges as Two Strains Combine, WHO Warns
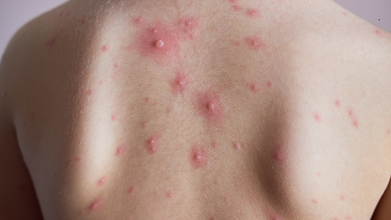
Credit: Canva
Two strains of the monkeypox virus (MPXV) have combined with each other to create a new version of the disease, prompting the World Health Organisation to raise an alarm.
According to scientists, the Ib and IIb of MPXV have mutated together, and a case has been found in the UK and India, respectively. The first case was detected in the UK with travel history to a country in South-East Asia, and the second in India, with travel history to a country in the Arabian Peninsula.
Further analysis of each case shows that the two individuals fell ill several weeks apart with the same combined strain. Both cases had similar reported signs and symptoms of the disease and neither experienced severe outcomes.
As of now, these are the only known cases of this version of the virus.
What Is Mpox?
Mpox (formerly monkeypox) is an infectious disease caused by the monkeypox virus, a member of the Orthopoxvirus genus. Symptoms usually appear 1-21 days after exposure, and the illness lasts 2–4 weeks. People are considered to be contagious until all scabs have fallen off.
Mostly based in Africa, it was discovered by captive monkeys in 1958, after whom the disease was named in 1970. However, the name attracted many racist comments, especially on social media, where people wrote “the disease of monkeys” and associated it with Africans.
Under WHO guidelines, the naming of diseases must not drive any unnecessary negative impact on trade, travel, tourism or animal welfare, and avoid offending any cultural, social, national, regional, professional or ethnic groups. Thus, the name monkeypox became the ‘m-pox’.
How Can You Detect Mpox?
There are signs and symptoms of M-pox. They start to show within seven to 14 days of being infected. Therefore, for about a week, a person may not know they have m-pox, and they can spread it by travelling.
The earliest signs are getting a fever, sweating and having chills through your body. Other signs involve rashes, which start from a distant rash on the face and spread throughout the body. These rashes can be in different forms, sometimes a flat lesion, bumps, boils or scabs.
Symptoms can also include swollen lymph nodes, migraine, muscle aches, fatigue, weakness and back pain.
How Do You Prevent Mpox?
This is a contagious infection and can spread by skin-to-skin contact. Here are some things you can do to safeguard yourself:- Restrict your movement by avoiding going out in public, meeting people and interacting with animals.
- Wear clothes that will prevent skin-to-skin infections.
- Hydrate yourself to get rid of the toxins from your body
- Check if you have received your smallpox vaccination
Doctors also prescribe medications like acetaminophen and ibuprofen to treat the pain and fever one may experience after being infected
© 2024 Bennett, Coleman & Company Limited

