- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
World Autism Awareness Day 2025: Theme, Significance, Origin, And Importance

Credits: Canva
Every year on April 2, World Autism Awareness Day is observed. This day raises awareness about autism spectrum disorder (ASD) which is a developmental brain disorder which impacts how a person perceives and socializes with others. This condition may cause problems in social interaction and communication. The condition may also include limited and repetitive patterns of behavior.
Origin of World Autism Day
It was started in 2007, when the United Nations General Assembly designated 2 April as World Autism Awareness Day. The UN worked to promote the full realization of human rights and fundamental freedoms for autistic individuals and ensured their equal participation in society.
In fact, over the years, progress has been made and driven in large by autistic advocates who have worked tirelessly to bring the lived experiences of autistic individuals to the forefront of global discussions.
The 2007 General Assembly resolution also highlighted the need to raise public awareness of autism.
Theme of World Autism Day
The theme for 2025 is "Advancing Neurodiversity and the UN Sustainable Development Goals (SDGs)". This goal highlights the intersection between neurodiversity and global sustainability efforts and showcases how inclusive policies and practices can drive positive change for autistic individuals worldwide and contribute to the achievement of SDG.
This year's theme and discussion also explores at the role of neurodiversity in shaping policies that promote accessibility, equality, and innovation across multiple sectors.
Significance of World Autism Day
The day aims to foster a better understanding of autism and the importance of early diagnosis and intervention. The day also tries to promote awareness and acceptance and create a more inclusive and supportive environment for individuals with autism.
What Is Autism?
As per the American Psychiatric Association, ASD is a complex developmental condition involving persistent challenges with social communication, restricted interests and repetitive behavior. While autism is considered a lifelong condition, the need for services and supports because of these challenges varies among individuals with autism.
As per the Centers for Disease Control and Prevention, an estimated one in 36 children have been identified with ASD.
Recent News Around Autism
Ramsey's Diagnosis: Recently, star of HBO's hit drama Bella Ramsey opened up about their autism diagnosis. They credited a crew member for recognizing the signs and symptoms.
Ramsey shared that they were diagnosed while filing the first season of the series.
“I’ve spoken a bit about neurodivergence before, but I always for some reason didn’t want to,” Ramsey admitted. “I got diagnosed with autism when I was filming season one of The Last of Us.”
While filming in Canada, a crew member who also has an autistic daughter noticed certain similarities in Ramsey's behavior and suspected that they too might be autistic. This observation was what prompted Ramsey to seek a formal psychiatric assessment, and thus leading to their diagnosis.
Ramsey also reflected on their childhood and described feeling out of place in school and finding comfort in the company of adults. They also recalled that they had experienced sensory sensitivities common among autistic individuals, such as heightened awareness of micro-expressions and body language. Ramsey also mentioned that filming in Canada's cold condition was also challenging as the heavy waterproof gear and thermals were required on the set. "It was too much stuff on my body," they shared talking about sensory discomfort.
ALSO READ: If Vaccines Don't Cause Autism-Here's What Does
CDC's Vaccine Study: The Centers for Disease Control and Prevention (CDC) is planning to examine potential link between vaccines and autism. This has all come up in the backdrop of the claims by the now Health and Human Services (HHS) Secretary Robert F Kennedy Jr, who has suggested the link between vaccines and autism. This has been backed by President Trump as well.
This originated from now debunked 1998 study, which was retracted after its author, Andrew Wakefield, was found guilty of professional misconduct and barred from practicing medicine in the UK. Despite decades or research no such link could be proven, however, the claim still continues in the political and public discourse.
Kennedy, who had long been anti-vaxxer, also made numerous claims, even though studies have long debunked this theory.
Metastatic Breast Cancer Awareness Day: Importance, Themes, And More

Credits: Canva
The entire month of October is observed as the Breast Cancer Awareness Month, and today, on October 13, the Metastatic Breast Cancer Awareness Day is observed.
What Exactly Does Metastatic Breast Cancer Mean?
Metastatic breast cancer, also known as stage IV breast cancer, is a form of the disease where cancer cells have spread from the breast to distant parts of the body, most commonly the bones, liver, lungs, or brain. While there is no cure, treatments like chemotherapy, hormone therapy, targeted drugs, and immunotherapy can help manage symptoms, slow the disease's progression, and improve quality of life. Symptoms vary depending on the area affected but can include bone pain, shortness of breath, fatigue, and headaches.
The day is specifically recognized in the US as a day to promote awareness around breast cancer. In fact, on this day 300 monuments across all 50 states lit up and a dozen other countries from around the world also light up in green, pink, and teal. These are the colors of breast cancer awareness ribbon.
Why Is It Important?
It is an international health campaign that aims to promote screening and reduce the risk of the disease. Breast cancer impacts around 2.3 million women worldwide.
Through this day and the entire month that is dedicated to awareness, people and organizations can:
- support people diagnosed with breast cancer, including those with metastatic breast cancer
- educate people about breast cancer risk factors
- encourage women to go for regular breast cancer screening starting at age 40 or earlier, depending on personal breast cancer risk
- raise money for breast cancer research
Metastatic Breast Cancer Awareness Day Importance
About 168,000 women in the US are estimated to have metastatic breast cancer. There are research and studies that show this number will rise to over 246,000 by 2030. Despite the growing numbers of people living with metastatic disease, most money for breast cancer research doesn’t go toward studying it.
Metastatic Breast Cancer Awareness Day thus seeks to educate the public about the challenges that people with metastatic breast cancer face and the need for more research, and more treatments — for this deadly disease.
Is There A Theme For Metastatic Breast Cancer Awareness Day?
While there is no specific theme for Metastatic Breast Cancer Awareness Day, the day focuses on early diagnosis and on the support group that many patients need once they are diagnosed.
Themes often emphasize the unique experiences of patients with metastatic disease and the urgent need for support and a greater understanding of this advanced stage of cancer.
In fact, this year's theme for the Breast Cancer Awareness Month is also 'Every Story is Unique, Every Journey Matters'. This emphasizes on personalized stories of the ones who battled or are battling cancer, as no two patients journey through cancer is the same.
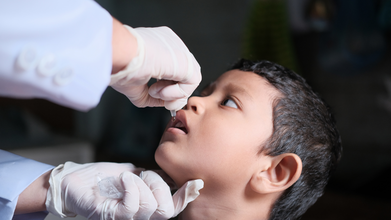
Pulse Polio Drive In Hyderabad To Target Over 500k Children, Details Inside
Credits: Canva
Telangana, a southern state in India has launched a Polio Drive from October 12 to 15 with Hyderabad administering oral polio drops to 5,17,238 children. This is part of Telangana's Pulse Polio Immunisation Drive, 2025. Hyderabad is one of six districts participating in this nationwide effort to prevent polio resurgence.
What Is Happening In Hyderabad?
Hyderabad has set up 2,843 vaccination booths which will operate from 7am to 6pm at health centers, schools, transport hubs, and high footfall areas.
Over 11,200 health workers, including ASHA and Anganwadi staff, will also conduct door-to-door visits from October 13 to 15, targeting 164 high-risk localities to ensure full coverage. Around 9.36 lakh households are expected to be reached during the campaign.
The drive is part of India’s ongoing national effort to maintain its polio-free status, especially amid reports of poliovirus cases in neighboring countries like Bangladesh and Pakistan. Officials have urged parents to ensure that children receive both required doses, stressing that even a single missed child can pose a risk to the wider community.
Parents can visit nearby booths or contact local health workers for information. Assistance is also available via helplines 1070 (State Control Room) and 1098 (Child Line) for guidance on immunisation schedules.
What Is Polio?
The World Health Organization (WHO) notes that it is a highly infectious disease caused by a virus that invades the nervous system and can cause total paralysis in matter of hours. The virus is transmitted by person to person spread mainly through the fecal-oral route, or less frequently, by a common vehicle, which could be either from contaminated food or water. The virus also multiplies in the intestine.
The National Institute of Health (NIH), US, notes that the virus responsible for causing Polio belongs to the Picornaviridae family.
Read: Polio Cases Reported In Pakistan And Nigeria, More Details Inside
Common Symptoms of Polio
The NIH notes that the maximum excretion of the virus is seen in 2 to 3 days prior and 1 week after appearance of symptoms. The spread is especially rapid in areas with poor sanitation, and among the nonimmune population. The rise in cases could be seen in summer months and temperate regions.
As per the WHO, the common Polio symptoms are:
- Fever
- Fatigue
- Headache
- Vomiting
- Stiffness of the neck a
- Pain in the limbs
The WHO also notes that 1 in 200 infections leads to intervisible paralysis, usually in legs, and among 5 to 10% of those paralyzed do not survive.
Who Are Most At The Risk Of Polio?
As per the WHO, Polio mainly affects children under 5 years of age, however, it can happen to anyone of any age who is unvaccinated and may have come in contract with the disease.
Is There Any Cure For Polio?
While there is no cure for Polio, it can very well be prevented. Polio vaccines, which can be administered multiple times, can protect a child for life. There are two vaccines available:
- Oral Polio Vaccine
- Inactivated Polio Vaccines
Tiger Woods, America's Top Golfer Undergoes A Surgery For Collapsed Disc, More Details Inside

Source: X
Tiger Woods, American professional golfer has announced that he underwent a back surgery on Friday. This was to addressed his collapsed disc in his spine.
The 15-time major winner shared on his social media that he had a lumbar disk replacement surgery after he experienced in his back. The operation went successful, he said. The 49-year-old is undergoing a back surgery for the second time in over a year. Earlier, he had a surgery done on his lumbar spine in September 2024 to relieve nerve impingement of his lower back.
A statement by Wood's X account read: “After experiencing pain and lack of mobility in my back, I consulted with Doctors and Surgeons to have tests taken. The scans determined that I had a collapsed disc in L4/5, disc fragments and a compromised spinal canal. I opted to have my disc replaced yesterday, and I already know I made a good decision for my health and my back. On Friday, Tiger underwent lumbar disc replacement surgery in his L4/5 Lumbar spine for lower back symptoms. The surgery was deemed successful and performed by Dr Sheeraz Qureshi and his team at the Hospital for Special Surgery in New York.”
Woods had earlier ruptured his left Achilles while training at home this year in March.
What Does It Mean To Have A Collapsed Disc?
Spinal discs sit between the 33 vertebrae that provide cushioning to vertebrae and absorb shock and pressure. They also help with flexibility, mobility and are made of tough outer layer of cartilage, surrounded with a soft jelly-like inner layer.
However as we age, our spinal disc also age with us. They become drier, stiffer, and less flexible over time, which makes it prone to injury and damage. This natural wear and tear can cause discs to tear or to slip out of place. A collapsed disc destabilizes the spine and hinders the discs’ ability to protect vertebrae.
Collapsed discs occur more frequently in cervical and lumbar spine. This is where vertebrae bear more body weight and have a wide range of motion, which makes them more prone to injury or damage.
What Are The Common Symptoms?
In a Lumbar Spine, the common symptoms of collapsed disc are:
- low back pain
- pain that radiates down the buttocks, thighs, legs and feet
- weakness and numbness or tingling in the back, legs, and feet
- reduced movement in the back
In Cervical Spine, this is what the symptom of collapsed disk feels like:
- neck pain
- pain that radiates down the shoulders, arms, hands, and fingers
- weakness and numbness or even tingling in arms, hands, and fingers
- reduced range of motion in the neck
What Could Be Causing Disc To Collapse?
Spinal Osteoarthritis: This happens when the cartilage that cushions the spine’s joints wears down, leading to pain, stiffness, and reduced movement. Over time, it can weaken nearby structures and cause the discs between the vertebrae to collapse.
Herniated Disc: A herniated disc occurs when the outer layer of a spinal disc tears, allowing the inner gel-like material to leak out. This can cause pain, pressure on nerves, or even break the disc into smaller pieces.
Degenerative Disc Disease (DDD): DDD refers to age-related wear and tear of spinal discs. It can lead to back pain, disc collapse, nerve compression, and spinal instability.
Other causes include traumatic injuries (from falls, accidents, or sports), repetitive strain (from heavy work or high-impact activities like running), and obesity, which adds pressure to the spine.
© 2024 Bennett, Coleman & Company Limited

