- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
World Yoga Day 2025: Theme, History, And Importance

Credits: Canva
Yoga is more than just a form of exercise—it’s a way of life. Rooted in India’s ancient spiritual traditions, yoga nurtures the body, sharpens the mind, and uplifts the soul. On June 21 each year, the world unites to celebrate International Yoga Day, recognizing the profound and lasting impact of this holistic discipline on global well-being.
Theme of 2025: “Yoga for One Earth, One Health”
The theme for International Yoga Day 2025 is “Yoga for One Earth, One Health”. As we mark the 11th year of this global celebration, the theme emphasizes the interconnectedness between planetary health and human well-being. It underscores the role of yoga in creating a more sustainable and mindful lifestyle that nurtures both our inner world and the environment around us.
Why This Theme Matters
At a time when the planet is grappling with climate change, rising mental health concerns, and lifestyle-related diseases, this theme reinforces the idea that personal wellness and ecological balance go hand in hand. Yoga encourages conscious living, reduced consumption, and mindfulness—all of which are key to healing both people and the planet.
Why International Yoga Day Is Celebrated
The purpose of International Yoga Day is to raise awareness about the many benefits of practising yoga. In a world grappling with stress, sedentary lifestyles, and disconnection from nature, yoga offers a much-needed balance. It brings together physical strength, mental clarity, emotional resilience, and spiritual peace—making it a truly integrative practice for modern life.
How It Came to Be
The idea for an international day to celebrate yoga was first proposed by Indian Prime Minister Narendra Modi during his speech at the 69th session of the United Nations General Assembly in September 2014. He described yoga as an “invaluable gift” from India’s ancient tradition, highlighting its role in promoting harmony between mind and body, and between humanity and nature.
The proposal received overwhelming support—175 member states co-sponsored the resolution, and on December 11, 2014, the UN officially declared June 21 as International Day of Yoga. The first celebration was held on June 21, 2015, in New Delhi, where nearly 36,000 people from 84 countries participated, setting two Guinness World Records.
Why June 21?
The date June 21 was chosen for its symbolic significance—it is the Summer Solstice, the longest day of the year in the Northern Hemisphere. This day is considered auspicious in many cultures and marks a transition to a more spiritually aware phase in the yogic tradition. It reflects the harmony between nature and human life, perfectly aligning with yoga’s core principles.
How India is Celebrating in 2025
This year, India is hosting 10 signature events, with ‘Yoga Sangam’ as the flagship initiative showcasing mass yoga demonstrations at 1,00,000 locations nationwide. Other events such as Yoga Bandhan, Harit Yoga, and Yoga Mahakumbh reflect the diversity of yoga practices and their relevance in different aspects of daily life.
New Weight Loss Pill Can 'Cut' Body Weight By 24% Without The Jabs, Says Study

Credits: Canva
New weight loss drug 'Amycretin' is cause for a breakthrough in the medical community, with research indicating it can assist people in losing a substantial percentage of their body weight in an unprecedented time. Released in The Lancet and at the American Diabetes Association's Scientific Sessions in Chicago, preliminary results indicate that this medication may surpass even the most powerful injections available today—and with the bonus of taking a simple daily tablet.
Although still in pre-stage trials, the outcomes are intriguing: amycretin could result in weight loss of as much as 24.3% in 36 weeks with a weekly injection, and more than 13% weight loss of the body within three months when administered orally in tablet form. These are higher outcomes than current GLP-1 receptor agonists such as Wegovy and Mounjaro, and ranks it as one of the most viable on the pipeline for anti-obesity drugs.
Amycretin is a product of Novo Nordisk, the manufacturer of popular GLP-1-based medications such as Wegovy and Ozempic. Amycretin is unique in that it has a dual mechanism of action. It acts not just on the GLP-1 receptor to reduce appetite and delay gastric emptying but also on the amylin receptor, adding to its effects of satiety and glucose control.
In a Phase 1b/2a trial with 125 patients, individuals given the maximum dose of amycretin injections (60mg weekly) saw an impressive 24.3% loss of body weight after 36 weeks. Metabolic marker and blood sugar improvements were also noted, putting amycretin on the map as both a weight control and metabolic health agent.
The parallel 12-week 144-patient tablet-based trial involved subjects taking the highest dose of 100mg a day, and they lost 13.1% of their body weight. This is especially heartening considering the practicalities and resource issues normally linked with injectable treatments.
Existing weight loss drugs such as Wegovy and Mounjaro, although effective, are normally in the form of weekly or every-other-week injections—more often than not under medical supervision. Apart from imposing additional burdens on already overwhelmed healthcare systems, this also discourages many prospective consumers.
A once-a-day pill version of amycretin might be a game-changer, providing a more convenient, scalable, and consumer-friendly option. Such ease might expand the availability of successful weight loss treatment, which nearly 40% of American adults rely on, as the CDC states.
In the UK, an estimated 1.5 million people are already using weight loss injections, many through private prescriptions. Starting next week, general practitioners in Britain will be authorized to administer these jabs under NHS guidelines, although the rollout is limited to just 220,000 people over three years and restricted to those with extreme obesity and multiple chronic conditions.
Are the Results Too Good to Be True?
Although the findings so far are promising, caution is called for by experts. The excitement over amycretin is comparable to the welcome that was given to previous GLP-1 agonists, but the drug's long-term safety and effectiveness must be proven.
The side effects noted, such as nausea, vomiting, and anorexia, were characterized as mild to moderate in both trials and generally resolved by the end of treatment. However, these are consistent with other GLP-1 drugs and may not be acceptable in all patients.
"There is a worry that some users, desperate for quick fixes, will take shortcuts through pain rather than consulting with healthcare professionals to get the optimal dosage," said Dr. Thiara, an assistant clinical professor of medicine at UCSF.
Further, questions are raised about the long-term maintenance of weight loss. As with other GLP-1-based treatments, sustained use seems to be required to sustain weight loss, maybe making amycretin a drug to be used for a lifetime.
Notwithstanding the promise, access is an obstacle. Most patients, especially in the United States, find it difficult to access GLP-1 medicines because of shortages, insurance restrictions, or out-of-pocket expenses. In some instances, individuals have to travel long distances simply to collect their medication.
In addition, the FDA only approves GLP-1 weight loss medications for people with a BMI of 30 or higher or a lower BMI with comorbidities like hypertension or Type 2 diabetes. Although some providers are willing to prescribe off-label, the most at-risk individuals usually encounter the most barriers to care.
Amycretin's formulation in oral form can fill this gap, providing a more convenient answer to widespread application, if and when it becomes approved for over-the-counter or widespread prescription use.
The development of drugs such as amycretin brings with it a larger conversation about the way society views and manages obesity. While these medications offer concrete solutions for weight, they also present ethical questions: Do these medications help fuel stigma toward individuals who exist in larger bodies? Are they perpetuating a culture of thinness at the expense of comprehensive health?
Nevertheless, for most patients and clinicians, health is still valued over looks. As more becomes known about how obesity is a multifaceted chronic illness with hormonal, psychological, and lifestyle components, instruments such as amycretin can provide much-needed relief and empowerment to afflicted individuals.
Early outcomes are encouraging, but further trials of greater size and duration are indicated. Researchers on the amycretin trials recommend increased research with a larger and more varied participant population to validate results and identify ideal dosing regimens, especially in individuals with conditions such as Type 2 diabetes.
The reports already published in The Lancet are early-stage trials, and amycretin has yet to become accessible to the public. If later phases confirm the drug's effectiveness and safety, amycretin could soon join or potentially even overtake the likes of Wegovy and Mounjaro in revolutionizing obesity treatment worldwide.
Amycretin is leading the charge in what could be a revolution in weight loss and metabolic wellness therapy. With both injectable and pill forms achieving record-breaking weight loss results, the drug represents a new front in the treatment of obesity—a public health epidemic that currently touches millions across the globe but as with every medical development, careful thought, stringent testing, and ethical use will be necessary to make certain the solution is not worse than it is better.
First Heatwave of 2025 Scorches UK, Northern Europe; Health Warnings Issued

Credits: Canva
Northern Europe is grappling with an intense early summer heatwave this weekend, with authorities in the United Kingdom and France issuing health alerts as temperatures soar well above seasonal norms.
The UK Met Office has forecasted highs of 34°C (93°F) in parts of eastern England on Saturday, marking a dramatic 12°C (22°F) increase above average for this time of year. The heat is part of a broader pattern affecting much of northern and western Europe, where unseasonably hot weather has settled in following a week of rising temperatures.
In France, the heat is even more severe. Meteo France, the national weather service, has predicted temperatures will reach 38°C (100°F) in parts of the western and southern regions of the country — particularly dangerous for vulnerable populations.
Heat Health Alerts Issued Across England
Responding to the escalating heat, the UK Health Security Agency (UKHSA) and the Met Office have jointly issued an amber heat health alert for the whole of England, in place through Monday morning. The alert specifically targets increased risks for older adults, especially those aged 65 and above, as well as individuals with chronic heart and lung conditions.
“Heat can result in serious health outcomes across the population, especially for older adults or those with pre-existing health conditions,” said Dr. Agostinho Sousa, head of extreme event health protection at the UKHSA. “It is therefore important to check on friends, family and neighbors who are more vulnerable and to take sensible precautions while enjoying the sun.”
Authorities are urging people to stay hydrated, avoid physical exertion during peak sunlight hours, and remain indoors where possible. The UK has increasingly been experiencing heatwaves in recent years, a trend that scientists say is likely to intensify due to climate change.
France Warns: No One Is Immune to the Heat
Meteo France has echoed similar concerns in its alerts for western and central France. The agency has cautioned that the current heatwave can pose health risks not only for vulnerable populations but also for healthy individuals, especially those exposed to prolonged sun or engaged in physical activity.
“Everyone is at risk, even healthy people,” the agency warned, underlining the importance of recognizing the early signs of heat-related illnesses like heatstroke and dehydration.
Real-Time Impact: Paris Air Show Attendees Seek Shade
The impact of the scorching temperatures was visible earlier this week at the Paris Air Show, held at Le Bourget airport north of the French capital. Aviation enthusiasts and industry professionals attending the event were seen clustering in the shade of aircraft wings — including that of a Boeing 777 — to escape the heat, as the mercury hovered in the low 30s°C (mid-80s°F).
The image has become symbolic of how Europe’s iconic summer events are being reshaped by climate extremes, with rising heat affecting not only public health but also tourism and outdoor activities.
Cooler Weather Expected Next Week
According to British forecasters, Saturday is expected to mark the peak of the heatwave. Temperatures are forecast to dip slightly on Sunday, followed by a gradual return to more typical early-summer levels by midweek.
While the immediate heat threat may ease in a few days, public health agencies are urging people not to drop their guard. “Heatwaves are becoming more frequent and more intense,” Dr. Sousa warned, “and our response must adapt accordingly.”
Chicken Alfredo Meals Recalled After Listeria Outbreak Across 13 States
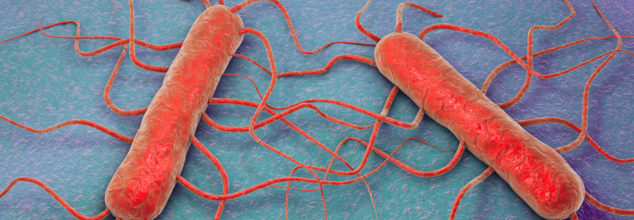
Credits: Canva
Premade chicken fettuccine Alfredo meals sold at Walmart and Kroger have been recalled following a multi-state listeria outbreak that has led to 17 illnesses, three deaths, and one pregnancy loss, according to the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS).
What’s Behind the Outbreak?
The affected products were made by FreshRealm, a Texas-based food company. FSIS reports that the same strain of Listeria monocytogenes linked to the outbreak was found during routine testing at a FreshRealm facility in March 2025. Investigators are still working to determine whether a specific ingredient in the Alfredo meals caused the contamination.
So far, cases have been reported across 13 states between August 2024 and May 2025.
Which Products Were Recalled?
The recall involves chicken Alfredo meals sold at Walmart under the Marketside brand and at Kroger under the Home Chef brand. Specific items include:
- 32.8-ounce trays of “Marketside Grilled Chicken Alfredo with Fettuccine”
- 12.3-ounce trays of “Marketside Grilled Chicken Alfredo with Broccoli”
- 12.5-ounce trays of “Home Chef Heat & Eat Grilled Chicken Fettuccine Alfredo”
Consumers can check for establishment numbers Est. P-50784, Est. P-47770, or Est. P-47718 inside the USDA inspection mark on the packaging. Only these specific products are part of the recall. Other FreshRealm meals are not affected, CNN reported.
What You Should Do
FSIS warns that contaminated meals may still be in home refrigerators or freezers. If you purchased any of these recalled products, do not eat them. Instead, throw them away or return them to the store where they were bought.
To protect against illness, premade meals like these should always be heated to at least 165°F. Using a food thermometer is the only way to ensure the food reaches a temperature high enough to kill harmful bacteria, including listeria.
What Is Listeria?
As per the Centers for Disease Control and Prevention (CDC), Listeria are bacteria or germs that can contaminate many food and those who eat can get infected with the bacteria. CDC mentions that it is rare, however, could be serious, though there exists steps to prevent this infection.
It is a foodborne illness caused by the bacteria L. monocytogenes. Symptoms include fever, chills, and headache. It can cause invasive illness and intestinal illness. It is also the third leading cause of deaths from foodborne illness in the US, with 1,600 people infected each year, out of which 260 die. It is especially dangerous for:
- Pregnant women: may lead to miscarriage, stillbirth, or premature delivery
- Older adults
- People with weakened immune systems
Symptoms can include:
- Fever
- Muscle aches
- Headache
- Stiff neck
- Confusion or loss of balance
- Convulsions
If you're in a high-risk group and experience symptoms within two months of consuming a recalled product, seek medical care immediately and mention possible listeria exposure. The illness is typically treated with antibiotics.
© 2024 Bennett, Coleman & Company Limited

