- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Cancer Risk and Your DNA: What’s Hereditary and What’s Not?

(Credit-Canva)
When we think about cancer risk, it’s natural to wonder, “is it genetic?”
The truth is, sometimes it is, but in many cases, cancer develops from a mix of lifestyle, environmental factors, and DNA changes that occur over a lifetime. Understanding the difference between inherited genetic risks and those acquired along the way can help people make smarter decisions about screening, prevention, and treatment, and empower families to take proactive steps for their health.
Inherited genes or life choices?
Cancer arises from a series of changes/mutations in cells that disrupt normal growth control. Many of these changes happen over a person’s lifetime, influenced by exposures (like tobacco, UV rays, infections), aging, and random DNA errors. These are called “somatic mutations” and occur in our tissues—they are not inherited, and are not passed to children.
By contrast, a smaller fraction of cancers are influenced by inherited mutations called “germline mutations”; these are changes in the DNA that you are born with, and are present in every cell of your body. These mutations can predispose someone to cancer by impairing DNA repair, controlling cell division, or through other mechanisms. Approximately 5–10% of all cancers are thought to have a strong hereditary component.
So, while your DNA can influence your cancer risk, most cancers don’t occur because of an inherited gene defect. And even when a germline mutation is present, environment, lifestyle, and chance usually play significant roles in whether cancer actually develops.
Recognizing hereditary cancer syndromes
When should we suspect hereditary cancers? Here are red flags:
A strong family history of cancer, especially the same type (e.g. multiple members with breast cancer, or several relatives with colon cancer).
- Early-onset cancer, e.g. diagnosis before the age of 50 or 40 years.
- Multiple primary cancers in the same person (e.g., ovarian + breast).
Rare cancers or specific tumor types tied to known syndromes (e.g. medullary thyroid cancer, male breast cancer, pancreatic cancer in some families).
Known syndrome features, such as colon polyps and colon cancer in Lynch syndrome.
In such cases, genetic testing can identify mutations in genes like BRCA1/2, Lynch syndrome genes (MLH1, MSH2, MSH6, PMS2, EPCAM), TP53, PALB2, and others. Identifying carriers has implications for targeted screening (e.g. colonoscopic surveillance or mammography at regular intervals), preventive surgery like mastectomy, and sometimes therapy in case cancer does develop.
How do hereditary mutations lead to cancer?
Imagine your cells are factories, following a strict set of instructions (your DNA). Inherited mutations can mean that a “safety check” is broken from the start. For example:
A mutation in the BRCA1 or BRCA2 genes weakens the cell’s ability to repair DNA. Over time, unrepaired damage accumulates, raising the risk of developing breast, ovarian, prostate, and pancreatic cancer.
Mutations in DNA mismatch repair genes (as in Lynch syndrome) allow errors during DNA copying to persist, boosting mutation load and increasing the risk of developing colon, endometrium, stomach, and other cancers.
But even when a high-risk mutation is present, cancer doesn’t appear overnight. Additional “hits”, or more mutations, microenvironment changes, hormonal exposures, or lifestyle factors need to typically accumulate before cells turn cancerous.
Why does hereditary information matter?
You might ask: if it’s a small percentage of cancers, does knowing about hereditary risk make a difference?
The answer is, yes, absolutely. Knowing your hereditary risk of cancer has some important benefits:
Prevention & early detection: If you carry a pathogenic mutation, you can undergo more frequent surveillance, chemoprevention (e.g. tamoxifen for breast cancer), or risk-reducing surgeries (e.g. prophylactic mastectomy or oophorectomy).
Therapeutic choices: Certain inherited mutations also influence how cancers respond to therapy. For example, PARP inhibitors are effective in tumors with BRCA-related homologous recombination deficiency (HRD). Thus, knowing that a patient has a germline BRCA mutation may alter drug selection.
Family risk & cascade testing: Identifying a hereditary mutation allows cascade testing, where close relatives can also get genetic testing done. This helps them understand risks and take prevention measures before cancer develops.
Clinical trial access: Many modern trials require knowledge of inherited DNA defects. Patients with known germline mutations may qualify for therapies designed precisely for those DNA repair vulnerabilities.
However, it is also important to understand that absence of a germline mutation does not mean absence of risk. Many cancers are driven purely by somatic mutations, and many hereditary variants remain undiscovered or classified as Variants of Uncertain Significance (VUS). Testing negative for known genes does not guarantee immunity.
Also, hereditary risk is not absolute: a person may carry a mutation but never develop cancer, due to protective factors like healthy lifestyle, background genetics, or luck. Interpretation must be done thoughtfully, ideally with genetic counselling.
Conclusion
The relationship between cancer risk and our DNA is not simple.While hereditary mutations play a role in a minority of cases, their impact on prevention, therapy, and family planning can be profound. Knowing whether cancer “came from your DNA” is often less important than using that knowledge wisely—both for patients and their relatives.
As we move deeper into the era of precision medicine, clinicians and patients alike should appreciate that hereditary and somatic worlds coexist, and that DNA insight is a tool—not a verdict.
Prenatal Vitamins vs Multivitamins: Experts Reveal What Women Need

Credit: Canva
While you may have seen multiple women, who are not pregnant, taking prenatal vitamins to improve their skin, hair and nail quality, experts say you should steer clear and only stick to multivitamins to ensure your body stays healthy all-year round.
Prenatal vitamins are daily supplements for women who are pregnant or trying to get pregnant. These supplements contain the vitamins and minerals you need to support healthy fetal development, according to the Cleveland Clinic.
Dr Rohan Palshetkar, Consultant IVF Specialist, Bloom IVF tells Healthandme: ""Prenatal vitamin should be taken three months at least prior to the pregnancy or basically whenever you begin to start planning the pregnancy, ensure that all your micro nutrients as well as your folic acid levels are up to the normal mark so as to ensure that your baby has a healthy growth"
But when your body doesn't need it, taking supplements could put you at risk over time.
Which Vitamins And Minerals Are Prenatal Vitamins Rich In?
As per the American College of Obstetricians and Gynecologists (ACOG), here are the most important nutrients prenatal vitamins are packed with:
Folic Acid: One of the most important prenatal nutrients, this B vitamin is important as it creates your baby's neural tube. This is the structure that eventually forms brain. As per the US Preventive Services Task Force, folic acid supplements significantly increase the growth of healthy neural tube. The American Academy of Pediatrics also notes that it helps the neural tube to protect from defects by 50 percent.
Iron: It supplies blood and oxygen to the fetus and also helps build the placenta. It also gives the mother extra blood volume that you need throughout pregnancy. Pregnant people are prone to anemia, this is why iron supplementation is a must.
Calcium: The most time spend in uterus for a baby is invested in building their bones and teeth, this is a Herculean task, and requires the mother to have plenty of calcium. If you don't have enough calcium, then your baby will utilise the calcium from your body, which could lead to temporary bone loss.
Certain prenatal supplements also contain other additives such as omega-3 fatty acids.
Why Is It Dangerous To Take Prenatal Vitamins
The main difference between a prenatal vitamin and a multivitamin is the concentration of folic acid and iron.
Ingesting enough folate from food or folic acid from supplements at the start of a pregnancy lowers the risk of certain birth defects. Along with this, iron supplements help the body make the extra blood cells needed during pregnancy.
The amount of folic acid suggested for people who are planning a pregnancy is 400 to 800 micrograms (mcg) a day. The amount of iron needed in pregnancy is 27 milligrams (mg) a day.
The typical amount of folic acid for an non-pregnant adult is 400 mcg a day. For iron, the typical daily amount is 8 mg for males and 18 mg for females.
Taking iron and folic acid at levels higher than the suggested amounts may bump people closer to the upper limit for these nutrients which can raise the risk of health problems.
Taking too much folic acid, especially over 1,000 mcg daily without a doctor's advice, can mask a vitamin B12 deficiency, potentially causing irreversible nerve damage and may lead to side effects such as bitter taste, nausea or sleep problems, with potential links to increased risks in pregnancy like autism or insulin resistance.
Eating too much iron, especially from supplements, can cause acute iron poisoning, leading to severe nausea, vomiting, abdominal pain, diarrhea and bloody stools. Long-term excessive intake causes iron to deposit in tissues, causing iron overload (hemochromatosis) that damages the liver (cirrhosis), heart (failure), and pancreas (diabetes) and may cause a bronze skin color.
How The Buajau Sea Nomads Evolved Beyond Human Limits

Credits: Wikimedia Commons
Bajau Sea Nomads have evolved beyond human limits. But how is that even possible? Remember Charles Darwin's theory of natural selection? The survival of the fittest? This is what kept the Bajau Sea Noamds going, and evolving.
The Bajau people are sea nomads who survive by collecting shellfish from the sea floor. They come from South-East Asia, and study shows that they have developed bigger spleen for diving. They are known as 'Sea Gypsies' and are known to hold their breath for over five minutes, whereas highly trained professionals can hardly hold their breath for three to four minutes. Bajau divers spend hours underwater for fishing. They are also the world's only community of self-sufficient sea nomads, reported The Guardian.
How The Bajau Sea Nomads Evolved: What Does The Study Say?

A study published in the academic journal Cell notes that the effect of their underwater lifestyle reflected on their biology. Their spleens were larger than those of other people from the region.
How The Bajau Sea Nomads Evolved: How Does A Spleen Work?
Tucked just beside the stomach, the spleen is roughly the size of a fist and usually flies under the radar. Its everyday job is to filter old red blood cells from the blood. But under the right conditions, it can also act like a built-in oxygen reserve.
This hidden ability is especially important for the Bajau people, often called “sea nomads,” who live across parts of the southern Philippines, Indonesia and Malaysia. Numbering roughly a million, the Bajau have relied almost entirely on the ocean for generations.
“For possibly thousands of years, they have been living on houseboats, travelling from place to place in the waters of South-East Asia and visiting land only occasionally,” said Melissa Ilardo from the University of Copenhagen, speaking to the BBC’s Inside Science. “Everything they need, they get from the sea.”
Read: A Genetic Disorder Caused This Indonesian Tribe To Have Sparkling Blue Eyes
How The Bajau Sea Nomads Have Evolved?
The Bajau are known for their extraordinary free-diving abilities. When diving in the traditional way, they spend up to eight hours a day at sea, with around 60 percent of that time underwater. Individual dives can last anywhere from 30 seconds to several minutes, often reaching depths of more than 70 metres.
What makes this even more remarkable is the equipment. Many Bajau divers use little more than a wooden mask or simple goggles and a weight belt. No oxygen tanks, no wetsuits, no modern diving aids.
This lifestyle prompted researchers to ask whether the Bajau body has adapted over time to support such extreme breath-hold diving.
According to Dr Ilardo, the spleen was an obvious place to look. Humans, like many marine mammals, have a natural “dive response” that kicks in when we hold our breath and submerge ourselves in water, especially cold water.
“When this response is triggered, your heart rate slows down,” she explained. “Blood vessels in your arms and legs constrict to preserve oxygen-rich blood for vital organs like the brain and heart.”
The final part of this response involves the spleen.
How The Bajau Sea Nomads Evolved: An enlarged Spleen Boosts Oxygen

The spleen acts as a reservoir for oxygenated red blood cells. When it contracts during a dive, it releases these cells into the bloodstream, giving the body a temporary oxygen boost.
“It’s like a biological scuba tank,” Dr Ilardo said.
For people like the Bajau, who dive repeatedly every day, this spleen contraction can make a crucial difference, helping them stay underwater longer and recover faster between dives.
In short, what seems like an ordinary organ plays an extraordinary role, revealing how the human body can adapt in remarkable ways to extreme environments.
A Child Dies Every Nine Minutes in India From Drug Resistance, Data Shows
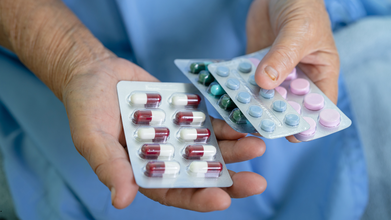
Credit: Canva
One child in India dies every nine minutes from an infection caused by antibiotic-resistant bacteria, as it becomes one of the top 10 global public health threats, experts warn.
Dr HB Veena Kumari of the Department of Neuromicrobiology, National Institute of Mental Health and Neurosciences, claims: "The Covid-19 pandemic has significantly contributed to rising antimicrobial resistance. The World Health Organisation projects that 10 million deaths will occur annually by 2025."
According to the National Foundation for Infectious Diseases, antibiotic resistance occurs when bacteria in the body learns to withstand and remain unaffected by the medicines (antibiotics) meant to kill them.
In such cases, doctors have to switch to different antibiotics, but these backup medicines might not work as well or might cause more side effects. Additionally, infections may also worsen over time as bacteria can become resistant to all available drugs.
Alarmingly is that these tough, drug-resistant bacteria can spread from one person to another, both in hospitals and at home.
According to Dr TS Balganesh, Gangagen Biotechnologies, nearly 36 percent of haemodialysis patients die from fatal infections, which is second only to cardiovascular diseases as a cause of death.
He tells Deccan Herald: "The risk for infective endocarditis in haemodialysis patients is approximately 18 times higher than in the general population and up to 58 percent of these episodes are caused by a bacteria named 'S aureus', with an in-hospital mortality of more than 50 percent."
What Does WHO Say?
One out of every six serious infections confirmed in labs worldwide last year could not be killed by the antibiotics meant to treat them.
Between 2018 and 2023, the problem of antibiotics failing (called resistance) got much worse. For many common types of germs, resistance went up by 5% to 15% every year. The growing inability of these essential medicines to work is a huge threat to people everywhere.
Which Antibiotics Are People Becoming Resistant To?
The WHO's latest report is the most detailed look yet at this issue. It reports on how much resistance exists across 22 different antibiotics, which include common drugs used to treat everyday illnesses. The report focused on eight common types of bacteria that cause things like:
- Urinary tract infections (UTIs)
- Stomach and intestinal infections
- Dangerous blood infections
- Gonorrhoea
Additionally, Dr Obaidur Rahman of Dr Ram Manohar Lohia Hospital has also warned that the country’s casual use of Azithromycin, sold under brand names such as Zithromax, Azee and Zmax, has worsened its effectiveness and pushed India closer to a major public health challenge.
A drug often prescribed for sore throats and upper respiratory tract infections, Dr Rahman noted that Azithromycin was once effective against Mycoplasma Pneumonia, a bacterium responsible for pneumonia in adults and children.
READ MORE: India’s New Antibiotic in 30 Years Offers Hope Against Antibacterial-Resistant Infections
However, this is no longer the case as India now shows an alarming 80 to 90 percent resistance to the drug when treating infections caused by this bacterium. A medicine that once addressed a wide range of respiratory problems is no longer reliable for many patients.
The surgeon has since urged people to avoid taking antibiotics without proper medical advice. Most seasonal respiratory infections resolve on their own, and unnecessary drugs only add to the resistance problem.
© 2024 Bennett, Coleman & Company Limited

