- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
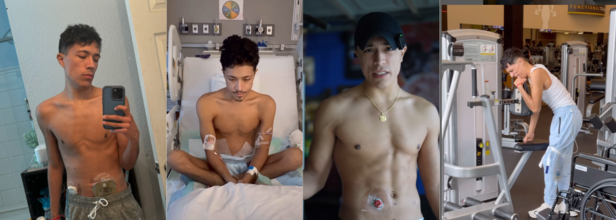
Fitness Trainer Shares About The Condition That Left Him With A Hole In His Stomach
Credits: Instagram
Samuel Richards, 23, a fitness trainer based in San Antonio, Texas lost around 50 pounds of his muscle mass. A fitness trainer who would go around flaunting his trained body was no longer in the condition to even stand up and go to the bathroom. He took to his Instagram to share his journey of what had happened to him and how from there he stood back up, worked on himself again, and is back to being a fitness trainer.
He shares, "I have something called ulcerative colitis and I have to live with this hole in my stomach, which is the small intestine sticking through."
What is Ulcerative Colitis?
As per the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), it is a chronic inflammatory bowel disease (IBD) in which abnormal reactions of the immune system cause inflammation and ulcers on the inner lining of your large intestine. This can develop at any age but is more common to have developed in ages between 15 and 30.
Symptoms:
The symptoms vary from person to person, however, some of the common symptoms include:
- Diarrhea
- Passing blood in your stool
- Abdominal pain
It could be treated with medicines to reduce inflammation in the large intestine and help bring on and maintain remission. In some cases, doctors recommend surgery to treat ulcerative colitis. In Samuel's case, it was the surgery that left a hole in his stomach.
Colon Removal Surgery
He shared how because of the inflammation in his colon he had to get it removed, he says, "This affects the colon and I don't have mine. Which basically means I don't have to do number 2 anymore, I kind of just empty that in my bag." So, how does all of this happen?
This is what brings us to the colectomy or the bowel resection surgery. It is a surgical operation to remove part or all of your colon, depending on the situation of the patient. Why is the pouch attached to the stomach?
After your bowels have been resected, your surgeon could join the severed ends right away. If this is not the possibility, your surgeon may do it later, or at times it is just not possible. This is when your colectomy ends with colostomy or ileostomy. This means if your bowels cannot be reconnected at the time of your surgery, your surgeon will create a stoma in your abdominal wall and redirect the upper portion of your intestines to the stoma.
The Journey Ahead
Samuel shared that experts still do not know why it happens, though it has now become common knowledge that it happens because of genetics and stress. However, nobody is yet able to pinpoint the exact reason. As per the NIDDK, while experts are not sure what causes ulcerative colitis, they think it is genes, abnormal immune reactions, the microbiome, and the environment that play a major role.
Samuel now goes about his day with a stoma and a colostomy bag, which collects the waste from his intestine. It was on December 26, 2023, when he was admitted to the hospital with his condition. He was bleeding and had to use the bathroom every 15 minutes. He was so weak that he could not even make it out of the bed. After his colon was removed, he was still weak, struggling to come to terms with what had just happened. However, he kept at it, from being a fitness trainer, he had no muscle mass left. In his videos where he documented his journey, one can clearly see the bones showing through his skin.
However, it is his perseverance along with the medical assistance that helped him to bounce right back.
Not Just For Rest, A Good Night Sleep Can Flush Out Dementia-Linked Toxins

Credits: iStock
Scientists have long been puzzled over how the brain clears away its own waste. Unlike the rest of the body, which relies on the lymphatic system to carry waste from cells into circulation, the brain appeared to have no such mechanism. That mystery shifted about 12 years ago when researchers discovered the glymphatic system, a network that acts as the brain’s built-in cleaning service.
The glymphatic system works by circulating cerebrospinal fluid (CSF) through the brain’s tissues. This fluid enters the spaces between brain cells, collects waste, and carries it out along large veins. In animal studies, particularly in mice, the system appears most active during sleep. That discovery suggested that sleep might be essential for brain detoxification, and disrupted rest could interfere with waste clearance.
Among the most important toxins flushed out by the glymphatic system is amyloid beta (Aβ), a protein that, when accumulated, forms sticky plaques in the brain. These plaques, along with tangles of tau protein, are a defining feature of Alzheimer’s disease—the most common cause of dementia worldwide.
The idea that better sleep helps the brain clean itself is more than a scientific curiosity. It may help explain why people who consistently struggle with poor sleep face higher risks of dementia.
In humans, levels of amyloid beta in cerebrospinal fluid rise during waking hours and drop during sleep, suggesting that rest is when the brain “takes out the trash.” In one striking experiment, researchers kept healthy adults awake for a single night. Just 24 hours of sleep deprivation increased amyloid beta in the hippocampus, the brain region essential for memory and one of the first to show damage in Alzheimer’s disease.
Still, questions remain. While several mouse studies indicate the glymphatic system is most active at night, other recent experiments suggest it may work differently depending on the time of day or even the species. The debate highlights how much more we need to learn about how this system functions in humans.
How Do Sleep Disorders Increase Risk of Dementia?
Not all sleep is equal. Short-term sleep loss is harmful, but chronic sleep problems can be particularly damaging to brain health.
Sleep Apnoea
Sleep apnoea, where breathing repeatedly stops and starts during sleep, deprives the brain of oxygen and fragments rest. Both oxygen deprivation and chronic sleep disruption are thought to contribute to toxin build-up in the brain. Importantly, studies show that patients treated for sleep apnoea—often with continuous positive airway pressure (CPAP) machines—see greater clearance of amyloid beta. This suggests that treatment may help restore the brain’s waste-disposal rhythm.
Insomnia
Insomnia, defined as difficulty falling or staying asleep, has also been linked to higher dementia risk. While the association is clear, the mechanism is less so. Does insomnia accelerate amyloid build-up? Could treatment reverse the trend? Researchers are only beginning to explore whether therapies—such as orexin receptor antagonists, a new class of sleep drugs—might improve toxin clearance.
Untreated sleep disorders don’t just leave you tired—they may be undermining your brain’s long-term health.
Can Sleep Prevent Dementia?
While early findings are promising, science isn’t yet ready to declare sleep a cure for dementia. What researchers do know is that sleep deprivation can rapidly alter amyloid levels in the brain, and chronic sleep disorders such as apnoea and insomnia are associated with a higher risk of developing dementia. Treating sleep apnoea appears to improve amyloid clearance, though evidence regarding the effects of insomnia treatment remains limited.
What remains uncertain is whether improving sleep directly reduces dementia risk. Large, long-term clinical studies are still needed to confirm the link. Researchers are actively pursuing this question, measuring proteins like amyloid beta and tau in blood and spinal fluid across sleep-wake cycles, in both healthy individuals and those with sleep disorders.
The global dementia burden is growing. Alzheimer’s and related dementias currently affect more than 55 million people worldwide, with cases expected to triple by 2050. While scientists race to develop new drugs, lifestyle measures—such as improving sleep—are emerging as powerful, accessible tools for prevention.
If better sleep helps the glymphatic system flush out harmful proteins, prioritizing rest may be one of the simplest ways to protect long-term brain health. That means:
- Maintaining a consistent sleep schedule.
- Addressing sleep disorders like apnoea with medical guidance.
- Seeking help if insomnia is chronic.
While the science continues to evolve, the advice remains practical: treat sleep as essential, not optional.
The glymphatic system is a reminder that the brain, like the body, needs maintenance. Just as poor diet, smoking, or lack of exercise take their toll, chronic sleep disruption may leave toxins lingering in the brain, setting the stage for cognitive decline.
The exciting part is that this field of research is still in its infancy. Scientists are mapping the biology of how the brain cleans itself and testing new ways to boost that process. Whether through targeted drugs, therapies for sleep disorders, or simply protecting natural sleep cycles, the future may bring strategies to slow or even prevent dementia.
'Breast Cancer Symptoms Don't Always Come As Lumps' Breast Cancer Surgeon Reveals 4 Key Points Everyone Should Know

(Credit-Canva)
Breast cancer is one of the leading causes of death for women all over the world. It is the most common cancer diagnosed in American women and a leading cause of cancer death in less developed countries. In India alone, cancer cases are projected to reach over 1.5 million by 2025.
Since this disease is so widespread, it's essential to be well-informed. Dr. Lauren Ramsey, a breast cancer surgeon, shared four important facts that she believes every woman should know. These tips, originally posted on her Instagram, provide crucial information for understanding breast cancer risk and detection.
Family History Is Not the Only Risk Factor
Only a small number of breast cancer cases—about 5-10%—are actually caused by genes you inherit, such as the BRCA mutation. This means that most breast cancers are not passed down through families.
Therefore, even if no one in your family has ever had breast cancer, it's still extremely important for you to get regular screenings, like mammograms. Relying only on family history can give you a false sense of security, so remember that breast cancer can affect anyone, and consistent check-ups are your best defense.
Look Beyond a Lump
Many people think that the only sign of breast cancer is a lump. However, this isn't true. It's really important to pay attention to other changes in your breasts, because they can also be a sign of cancer.
Be on the lookout for things like changes in your skin, such as redness or dimpling (like an orange peel). Other signs can include swelling, a new pain that doesn't go away, or any unusual discharge from your nipple. Knowing what your breasts normally look and feel like is key to spotting these more subtle changes early.
Breast Density Affects Detection
Breast density refers to how much fibrous and glandular tissue a person has compared to fatty tissue. Many people, especially younger women, have naturally dense breasts. While this is completely normal and not a health problem on its own, it can make it harder for doctors to see breast cancer on a standard mammogram.
That's because both dense tissue and tumors appear white on a mammogram, making it difficult to tell them apart. If you have dense breasts, your doctor might recommend extra tests, like an ultrasound or an MRI, to get a clearer picture. You can ask your doctor about your breast density after your mammogram.
Lifestyle Changes Can Make a Difference
The healthy habits you often hear about—like eating well and exercising—are not just a suggestion; they can actually lower your risk of developing breast cancer. Making simple changes can have a big impact. Try to limit the amount of alcohol you drink and reduce your intake of processed foods, which are often high in sugar and unhealthy fats.
At the same time, try to be more physically active every day. Even a short daily walk can help. These positive choices are a great way to take control of your health and reduce your risk. This information is shared with care, so everyone can be empowered with knowledge about their health.
Ebola Outbreak In Africa: 15 Dead, 28 Suspected Cases Raise Alarms

(Credit-Canva)
Health officials in the Democratic Republic of the Congo (DRC) have announced a new outbreak of the Ebola virus in Kasai Province. As of September 4, 2025, there have been 28 suspected cases and 15 deaths, including four health workers. The outbreak is affecting the Bulape and Mweka health zones.
The alarm was first raised when people in the area started showing a mix of worrying symptoms, including fever, vomiting, diarrhea, and bleeding. These symptoms are tell-tale signs of a serious illness. To find out what was causing it, officials took samples and sent them to a lab in Kinshasa, the country's capital. The lab results came back on September 3rd, confirming the cause was the Ebola Zaire virus.
What Is Ebola Virus?
According to World Health Organization (WHO), Ebola is a very serious and often deadly illness in people. It's caused by several different viruses, with the most common being the Ebola virus, which has led to large outbreaks. The chance of a person dying from Ebola is about 50%, but this number has varied widely in the past, from 25% to 90%.
To ensure safety, a team of experts from WHO, and the DRC's own rapid response unit has been sent to the area. Their main job is to quickly find sick people and get them care, stop the virus from spreading in hospitals, and teach local communities how to protect themselves.
The WHO is also sending a massive delivery of two tons of medical supplies, including protective gear for health workers and a special mobile lab. This is a big help, especially because the area is hard to get to, with few roads and limited air travel.
What is The First Symptoms of Ebola
The symptoms of Ebola usually start to appear 8 to 10 days after a person has been in contact with the virus, but it can take anywhere from 2 to 21 days. At first, the symptoms are similar to common illnesses and are often called "dry" symptoms:
- Fever
- Severe headache
- Aches and pains in muscles and joints
- Feeling very weak and tired
- Sore throat
Later Symptoms
As the illness gets worse, usually after four or five days, the symptoms change and become more severe. These are known as "wet" symptoms:
- Loss of appetite
- Unexplained bleeding
- Stomach-related problems like nausea, vomiting, abdominal pain, and diarrhea.
How Do You Treat and Control Ebola Virus?
While there's no guaranteed cure, getting early and strong medical care—like staying hydrated and treating symptoms—can greatly increase a person's chances of survival. Right now, approved vaccines and treatments are only available for the most common type of Ebola (Ebola virus), but scientists are working on others. To stop an outbreak from spreading, health officials use a mix of strategies:
- Taking care of the sick in a way that provides a lot of support.
- Preventing infection in hospitals and communities.
- Finding and tracking everyone who might have been in contact with an infected person.
- Quickly testing samples in labs.
- Ensuring safe burials for those who have died.
- Using vaccines when available.
- Educating the public on how to stay safe.
How Is The Ebola Outbreak In Kasai Being Dealt With?
The DRC is well-prepared to fight this outbreak. The country has a supply of treatments and 2,000 doses of the Ervebo Ebola vaccine. This vaccine is very effective against this specific type of Ebola. The doses, which were ready and waiting in Kinshasa, are now being sent to Kasai.
They will be used to protect people who were in contact with infected individuals and to vaccinate the doctors and nurses on the front lines. The country has a lot of experience fighting Ebola—this is its 16th outbreak since the virus was first found in 1976. That experience will be a huge advantage in bringing this new outbreak to an end.
© 2024 Bennett, Coleman & Company Limited

