- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Have You Ever Felt Dizzy, Lightheaded When You Stand Up? Here's What It Means

Credits: Canva
Think about that fleeting moment when you get up after sitting or lying down—your head spins, your heart pounds, maybe you feel lightheaded or nauseated. If this scene has become all too familiar, you might be dealing with postural orthostatic tachycardia syndrome—POTS. It’s rare, but for the 1–3 million people in the U.S. who have it, it’s daily life. Now, a heart failure drug is showing real promise in taming the symptoms.
Ivabradine isn’t new—it’s been used for years to manage chronic heart failure, slowing the heart without dropping blood pressure. But a new pilot study, published in the Journal of Cardiovascular Pharmacology, suggests this drug might be a breakthrough for POTS patients. Researchers from UVA Health and Virginia Commonwealth University treated 10 young adults (average age 28, most of them women) with the drug. Normally, when these patients stood, their heart rates surged by around 40 beats per minute. After ivabradine? The spike shrank to only 15 bpm. And symptoms like faintness dropped by nearly 70%, chest pain by 66%—the difference wasn’t just physiological, it was life-changing.
Dr. Antonio Abbate from UVA Health called the findings compelling: cutting heart rate alone—without affecting blood pressure—appeared to break the chain of symptoms. “The inappropriate increase in heart rate is exactly why patients feel sick,” he said.
UVA Health Newsroom
What Is POTS?
Postural orthostatic tachycardia syndrome may sound technical, but its components describe the experience: "postural" (related to posture), "orthostatic" (standing upright), "tachycardia" (a fast heart rate), and "syndrome" (a bundle of symptoms). When someone with POTS stands, their autonomic system fails to constrict blood vessels effectively. The result? Blood tanks into the legs, the heart overcompensates, and you get hit by symptoms: dizziness, pounding heart, fatigue, brain fog, chest discomfort, sweating, nausea—anything but ordinary.
This isn’t a heart-muscle issue or a brain problem: it’s more like a software glitch in how your body regulates itself. It often affects young women between 15 and 50 and can stem from triggers like infections, trauma, pregnancy, or autoimmune diseases.
The recent UVA pilot study isn’t standalone. Earlier research supports the same direction. A 2017 retrospective study of 49 patients—almost all women—found 88% saw palpitations improve and 76% felt less lightheaded, with heart rates dipping and no significant change in blood pressure.
Then a 2021 randomized, placebo-controlled crossover trial—including 22 adults with hyperadrenergic POTS—took it further. The results showed substantial heart rate drops, improved physical and social quality of life, and even reduced norepinephrine levels (the stress hormone that tends to over-react upon standing). None of the participants developed dangerously low blood pressure.
And even earlier studies, including student-case reports and case series, all support the conclusion: ivabradine reduces heart rate without bringing blood pressure down—and that matters because traditional beta blockers can drop both, making some patients feel worse.
How Ivabradine Interrupts the Vicious Vagus Loop?
Here’s what researchers suspect is happening behind the scenes: when someone with POTS stands, the body overreacts with a surge of norepinephrine—our classic fight-or-flight hormone. The heart races, the brain kicks into panic mode, symptoms amplify, and the loop perpetuates itself. Ivabradine, by slowing the heart without altering blood pressure, effectively breaks that cycle at the source. Patients stop spinning, both literally and metaphorically.
What You Should Know POTS?
It's worth noting that these are still early results. The studies are relatively small, but statistically compelling. There's enough here, though, to encourage more formal trials—and for doctors and patients to take notice.
If POTS symptoms sound familiar—if you get faint when you stand, your heart races, and doctors struggle to pinpoint the cause—ivabradine may be a conversation worth having. It’s not a universal cure, but it’s different from other treatments. Rather than forcing blood vessels to tighten or increasing blood volume, it focuses squarely on the heart rate itself.
POTS has always been a misunderstood syndrome—a tricky physiological dance that leaves patients frustrated and clinicians unsure. But treating the pulse directly, instead of chasing blood pressure or fluid levels, looks like a game changer. Ivabradine isn’t a cure-all, but it's poised to offer relief where little existed before.
For anyone sick of dizzy spells, pounding hearts, or unexplained fatigue whenever they stand, it’s time to explore if this one medication could be the difference between feeling trapped and regaining control.
PCOS Could Be Raising Your Risk of Diabetes, Here’s The Hidden Link

Credits: Canva
Polycystic Ovary Syndrome (PCOS) is a common condition marked by the development of small cysts on the ovaries. This can interfere with ovulation, disrupt menstrual cycles, and impact fertility. Interestingly, many individuals with PCOS also experience insulin resistance, a condition where the body produces insulin, a hormone that regulates blood sugar but cannot use it effectively.
This raises concerns about a potential connection between PCOS and diabetes. We got in touch with Dr Tripti Sharan, Director of Obstetrics and Gynaecology at BLK-Max Super Speciality Hospital, explaining how PCOS may increase the risk of developing type 2 diabetes.
How PCOS Can Increase Diabetes Risk
Dr. Tripti explains that in PCOS, the ovaries do not function normally. Follicles are often stuck at different stages of development, giving the ovaries their characteristic “polycystic” appearance. However, the ovaries are not the underlying cause; they are affected by conditions like insulin resistance or hypothyroidism.“Insulin resistance is often driven by genetics, obesity, and lifestyle factors,” Dr Tripti notes. “To maintain normal blood sugar, the body produces more insulin. Excess insulin then affects the ovaries, causing them to release higher levels of male hormones. This can lead to irregular periods, acne, abnormal hair growth, and sometimes infertility.”
If these factors are left unaddressed, insulin resistance can worsen. Over time, the body struggles to regulate blood sugar effectively, potentially leading to diabetes.
Risk Factors for Diabetes in Women with PCOSPCOS is often seen as a pre-diabetic condition. Without proper management, it can progress to diabetes. Dr Tripti highlights the importance of lifestyle measures, such as maintaining a healthy weight, eating a balanced diet, managing stress, and adopting a structured daily routine.
ALSO READ: PCOS Awareness Month: 5 Lifestyle Tips To Manage PCOS According To Gynecologist
PCOS is also linked to other health concerns, including high triglycerides, fatty liver, endometrial hyperplasia, and high blood pressure. Factors such as obesity, inactivity, chronic stress, high cholesterol, pregnancy, aging, smoking, vitamin D deficiency, and misuse of steroids can further increase diabetes risk in women with PCOS.
Symptoms Indicating Higher Diabetes Risk
Signs that may indicate a higher risk of developing diabetes in PCOS include poor weight control, fatty liver, elevated lipid levels, and hormonal imbalances like excess androgens. These factors point to worsening insulin resistance, which significantly raises the likelihood of developing type 2 diabetes.Preventive Measures and Lifestyle Tips
Dr. Tripti recommends several strategies to reduce the risk of diabetes in women with PCOS:- Maintain a healthy weight
- Engage in daily physical activity, regardless of body size
- Manage stress effectively
- Prioritize sufficient sleep
- Address hormonal imbalances under medical supervision
- Treat thyroid disorders
- Monitor and control lipid levels to prevent cardiovascular issues
- Ensure pregnancies are planned and screened for diabetes
ALSO READ: Lori Harvey Opens Up About Endometriosis Journey, Shares What Brings Her Relief
She also advises focusing on a balanced diet rich in fiber, fruits, and vegetables, especially those with skins, which have a lower glycemic load. Adequate calcium and vitamin D intake are important, and regular exercise, including cardio, strength training, stretching, and relaxation practices like yoga and meditation can help manage both PCOS and diabetes risk. Additionally, avoiding smoking and limiting alcohol supports overall health.
Wellness Report: 1 In 10 Employees Sleep-Deprived, Young Workforce Faces Rising Health Risks

Credits: Canva
In today’s hyper-connected, always-on work culture, long hours and erratic shifts are quietly eating into one of the most essential pillars of health.. A new study by Truworth Wellness, India Workplace Wellbeing Report 2025: From Access to Outcomes, reveals how sleep deprivation, chronic diseases, and poor preventive care are undermining employees during their prime working years.
Sleep Deprivation: A Silent Productivity KillerThe report, based on health data from over 46,000 employees across industries, found that nearly 1 in 10 workers suffer from sleep disorders, most of them between ages 23 and 39. This lack of rest translates to 11.3 lost workdays per employee every year, costing companies an estimated ₹2.1 lakh annually, as per the study.
Chronic Conditions Striking Earlier
Cardiac issues, diabetes, and thyroid disorders are no longer diseases of old age. They are increasingly showing up in employees under 40, adding long-term pressure on both workers’ health and company healthcare costs.Obesity and the Prevention Gap
Obesity is emerging as a ‘gateway’ condition, with 14% of employees obese, 71% of them in the 25–35 age bracket. Despite being the most vulnerable, younger employees are also least likely to undergo preventive screenings, delaying early detection of high-risk conditions.Poor Sleep Is Leading To Poor Mental Health
The report also warns of the mental health toll of sleeplessness. Chronic fatigue, stress, and unmanaged conditions fuel declining resilience and rising emotional distress at work. As Rajesh Mundra, Founder and Executive Chairman of Truworth Wellness, notes, “Wellness can no longer be a checkbox activity, it must be embedded as a strategic business priority.”ALSO READ: High, Low, or Normal? A Simple Guide To Understanding Blood Pressure Readings
How Companies Can RespondTo tackle the crisis, the World Health Organization (WHO) recommends:
- Training managers to recognize and respond to emotional distress.
- Mental health literacy programs to reduce stigma and raise awareness.
- Stress management and lifestyle interventions such as physical activity and psychosocial support.
The Business Case for Better Sleep
Companies that invest in outcome-driven wellness programs already see 28% fewer sick leaves, 26% lower healthcare costs, and 11% higher revenue per employee. For every rupee spent, organizations save ₹289 in healthcare costs and ₹241 through reduced absenteeism.A Wake-Up Call for Corporate Industries
As sleep deprivation silently chips away at productivity, Indian businesses have an opportunity to treat wellness not as an add-on but as a strategic advantage. By tackling sleep, stress, and preventive care, organizations can build healthier, more resilient, and ultimately more productive workplaces.ALSO READ: Prediabetes: How To Spot the Silent Warning Signs Before It Turns Into Type-2 Diabetes
By moving beyond generic wellness programs and focusing on measurable outcomes, Indian organizations can turn employee health into a strategic advantage. Embedding sleep, stress management, and preventive care into workplace wellness not only improves productivity but also builds a resilient workforce. The Truworth Wellness India Workplace Wellbeing Report 2025, conducted in collaboration with People Matters, highlights these insights and provides a roadmap for companies to act before small health risks escalate into costly chronic conditions.
High, Low, or Normal? A Simple Guide To Understanding Blood Pressure Readings
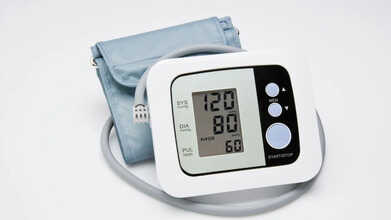
Credits: Canva
Half of all American adults have high blood pressure, also called hypertension, yet many don’t even know it. High blood pressure occurs when blood flows through your arteries at higher-than-normal pressures. Recently, cases of high blood pressure have been rising rapidly in the U.S. and globally. As these numbers climb, regularly checking your blood pressure becomes essential.
According to the 2025 American Heart Association Statistical Update, nearly half of U.S. adults, around 122 million people, have high blood pressure, a leading preventable cause of heart disease, stroke, and early death. Shockingly, only about one in four have their condition under control. To help you stay on top of your numbers, here’s a detailed guide to understanding blood pressure.
Blood Pressure Readings ExplainedBlood pressure is written as two numbers separated by a slash, such as 120/80 mm Hg, which can be read as “120 over 80 millimeters of mercury.”
The first number, or systolic pressure, measures the force of blood against artery walls when the heart pumps.
The second number, or diastolic pressure, measures the pressure when the heart rests between beats.
ALSO READ: World Lung Day 2025: How Strong Are Your Lungs? Do These Tests To Find Out
Blood pressure naturally changes throughout the day depending on activity, stress, and other factors. A healthy reading is less than 120/80 mm Hg. Blood pressure is considered high when systolic readings are 130 mm Hg or higher or diastolic readings are 80 mm Hg or higher.
New Blood Pressure Guidelines by the American Heart Association
In August 2025, the AHA and ACC updated blood pressure guidelines to emphasize early intervention and personalized care. Key points include:- Earlier Treatment: People with a 10-year cardiovascular risk of 10% or more are advised to start medication along with lifestyle changes.
- Personalized Risk Assessment: Doctors may use the PREVENT™️ calculator to estimate 10-year risk of heart attack, stroke, or heart failure.
- Expanded Lab Testing: Tests such as the urine albumin:creatinine ratio help tailor treatment plans.
- Lifestyle First: Limit sodium intake to 1,500 mg per day, maintain a healthy weight, and stay physically active.
- Alcohol Limits: Men: 2 drinks/day; women: 1 drink/day.
Blood Pressure Monitoring
The American Heart Association recommends that everyone with high blood pressure monitor their readings at home. Home monitoring helps healthcare professionals track whether treatments are effective and can also help confirm a diagnosis of high blood pressure. However, it does not replace regular doctor visits, and you should never stop taking prescribed medication without consulting your healthcare provider, regardless of your home readings.ALSO READ: This Small Mistake Can Change Your Blood Pressure Reading, According To Doctor
Home monitoring is particularly important for:
- Anyone already diagnosed with high blood pressure.
- People starting or adjusting high blood pressure treatments, to see if they are working.
- Individuals who require closer monitoring, especially those with risk factors for high blood pressure or related health conditions.
© 2024 Bennett, Coleman & Company Limited

