- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
1.3 Million Urged To Get RSV Vaccine- Will It Really Protect You Or Just A Scare?
Image Credit: Canva
While RSV cases still represent a major health threat, particularly for elderly people, the National Health Service (NHS) has recently made an emergency call for some 1.3 million people between the ages of 75-79 to receive the jab. But is the RSV vaccine actually the protection it's cracked up to be, or is it just another health panic? Here's what you should know about RSV, the vaccine, and whether you should get the vaccine.
RSV is a highly infectious virus that targets the respiratory system, typically causing symptoms like a cold in most people. Although young, healthy adults tend to recover swiftly, the virus can be lethal for older individuals, babies, and those who have compromised medical conditions.
RSV is frequently confused with a seasonal disease, occurring in the highest number in winter months, but it is continuously present throughout the year. In critical conditions, it may develop into pneumonia, worsening chronic lung disease, and even mortality. The statistics indicate that RSV causes about 60,000 to 160,000 admissions and 6,000 to 10,000 deaths each year in adults aged 65 years and older.
Why Is the NHS Asking Older Adults to Vaccinate?
The NHS started administering RSV vaccines on September 1, 2024, which was a major leap in the battle against respiratory illness. To date, almost 1.5 million individuals have been vaccinated, but there is still a vast number of unvaccinated people at risk.
Steve Russell, NHS National Director of Vaccinations and Screening, highlighted the jab's importance, saying, "RSV is not a winter bug. We do see cases rise in winter, but it can happen throughout the year and can put older people in hospital." He also encouraged all those who are eligible to book their vaccination appointments to lower their risk of serious illness.
How Effective Is the RSV Vaccine?
Two key vaccines—AREXVY (developed by GSK) and ABRYSVO (developed by Pfizer)—have been crafted for older populations. In contrast to COVID-19 vaccines based on mRNA, these RSV vaccines function on the principle of delivering an inactive RSV protein to the body. This signals the immune system to identify the virus and become immune to the virus in repeat exposures.
It has been indicated through a research published in The Lancet that the initial year of RSV vaccination could spare:
- Up to 2,500 hospitalisations
- 15,000 general practitioner visits
- 60,000 cases of RSV-related illnesses in older adults
The Centers for Disease Control and Prevention (CDC) also recommends that adults aged 75 and older, along with those 60-74 who have underlying health conditions, receive the RSV vaccine. For those who were vaccinated last year, the CDC advises that a booster is unnecessary for the current season.
Should Pregnant Women and Infants Get Vaccinated?
Pregnant women can also get the RSV vaccine from 28 weeks, since RSV is a major cause of infant death. Almost 150,000 pregnant women have already been vaccinated since September, providing newborns with protection from birth to six months of age. The vaccine gives essential immunity during the crucial early months when babies are at their most susceptible.
The CDC has also approved another preventive option: monoclonal antibody injections for infants. These offer comparable protection against serious RSV infection in newborns and high-risk infants.
Potential Side Effects
Though RSV vaccines are effective, they do pose some risks. The U.S. Food and Drug Administration (FDA) mandates a warning label for AREXVY and ABRYSVO, notifying patients of a slight increased risk of Guillain-Barré Syndrome (GBS). GBS is an infrequent neurological disease that results in muscle weakness and paralysis, though its precise association with the RSV vaccine is unclear.
Other frequent side effects are:
- Mild fever
- Fatigue
- Injection site swelling or pain
- Headache
While these are possible side effects, top health professionals contend that the advantages far outweigh the risks, especially for seniors with existing health conditions.
Is This Just a Health Scare Or Is the Vaccine Worth It?
With each new vaccine introduction, there are questions of whether the push for immunization is warranted or if it's merely a reactionary health scare. With RSV, the evidence overwhelmingly supports vaccination as a preventive strategy.
Public health authorities maintain that the extremely high number of hospitalizations and fatalities in older adults related to RSV infection supports population immunization. The vaccine is not only preventive against mild, cold-like disease—it shields from severe complications such as pneumonia and lung disease worsening.
Should You Get the RSV Vaccine?
If you're 75 and above—or 60 to 74 with existing medical conditions—the RSV vaccine is strongly urged. Here's why:
- Severe complications are risked by high-risk individuals – Older adults, particularly those with heart or lung disease, are far more likely to be hospitalized by RSV.
- The vaccine works – In clinical trials, it has shown significant reduction in severe illness, hospitalization, and death.
- One dose gives immunity for a minimum of two years – In contrast to annual flu shots, the RSV vaccine provides immunity that lasts longer.
While skepticism around vaccines is understandable, particularly in a post-pandemic world, RSV is a proven threat to vulnerable populations. The NHS’s call for 1.3 million older adults to get vaccinated is not an overreaction—it’s a proactive step in safeguarding public health.
If you’re eligible, contact your GP today to schedule your vaccination. The protection it offers could mean the difference between a mild seasonal illness and a life-threatening complication.
RFK Hires Autism Skeptic To Look Into CDC Autism Data

Credits: MedPageToday
Dr. David Geier, a controversial orthopedic surgeon and known vaccine skeptic, is attempting to revisit long-debunked claims linking vaccines to autism. Recently hired by Health Secretary Robert F. Kennedy Jr., Geier is now reviewing historical safety data to investigate whether government agencies concealed crucial information.
Accessing CDC's Vaccine Safety Database
Geier is reportedly seeking access to the U.S. Centers for Disease Control and Prevention’s (CDC) Vaccine Safety Datalink (VSD), a repository of vaccine safety records from millions of patients. The VSD is maintained by a dozen major healthcare systems, each controlling its own data. Full access has always been tightly controlled due to privacy and misuse concerns.
Geier previously accessed the database in 2004 and 2006. However, according to The Wall Street Journal, CDC officials revoked his access both times, alleging he had misrepresented his research intentions.
Despite this, Geier is once again pushing to analyze the data. It remains unclear if access has been granted. A spokesperson for the U.S. Department of Health and Human Services (HHS) stated that the department intends to take a “fresh look at all data including old data,” and emphasized that they would “follow the science—wherever it leads.”
Scientific Community Raises Concerns
The scientific and public health communities have expressed concern over Geier's involvement. “He has no record in the scientific community of doing valid work,” said Dr. Walter Orenstein, former director of the CDC’s immunization program.
Geier and his late father, geneticist Mark Geier, have long promoted the theory that vaccines cause autism—an idea widely discredited by the medical community. The pair also introduced a controversial treatment using hormone-blocking drugs, which resulted in the revocation of Mark Geier’s medical license and disciplinary actions against David Geier for practicing medicine without a license.
At a 2015 conference, Geier defended his position, claiming the scientific community dismisses their findings without proper consideration. “They think that [the vaccine's link to autism has] been completely debunked,” he said at the time.
Kennedy's Role and the Larger Context
Robert F. Kennedy Jr., a vocal critic of vaccine mandates, has clarified that Geier will not lead autism research. Instead, his focus will be on identifying any possibly overlooked or hidden data within the CDC’s database.
“There has been a lot of monkey business with the VSD,” Kennedy stated in a previous congressional appearance.
Kennedy, who once authored a now-retracted Rolling Stone article alleging a vaccine-autism cover-up, cited a 2000 CDC conference that explored preliminary data on thimerosal—a mercury-based preservative once used in vaccines. Though early findings prompted questions, later analysis confirmed no link between thimerosal and autism. Thimerosal was removed from most vaccines in 2001, and final results were published in 2003.
Exploring Autism Causes and Rising Rates
While Geier conducts his review, the U.S. Department of Health and Human Services is separately examining rising autism rates. The CDC now estimates that 1 in 31 eight-year-olds in the U.S. were diagnosed with autism in 2022. Experts attribute the increase to a combination of better diagnostic methods, genetic factors, and increased awareness.
The National Institutes of Health (NIH) also plans to fund research into other possible causes, such as environmental toxins and diet. Meanwhile, Children's Health Defense, a nonprofit formerly led by Kennedy, is hosting an online event this week centered on what it calls the “autism cover-up.”
Covid-19 Active Cases Cross 7,100 In India But Signs Point To A Slowdown—How To Stay Protected Now?

India is again facing a concerning but seemingly controlled increase in Covid-19 cases. As of June 13, 2025, the active case count of the country is 7,131, as per the Ministry of Health and Family Welfare. Although new daily cases increased by 33 and three more deaths were added—two in Maharashtra and one in Madhya Pradesh—the overall trend indicates a potential plateau and even a slight dip, which gives optimistic hopes.
The most recent state-by-state Covid-19 dashboard published by the Ministry of Health and Family Welfare indicates remarkable regional variations in infection incidence in India. Kerala remains to have the highest burden, with 2,055 active cases, out of which 110 were new cases in one day. Gujarat is the second worst-affected state with 1,358 active cases, which saw a steep daily jump of 77 cases. Other majorly affected states are West Bengal, with 747 active cases; Delhi, with 714 cases; and Maharashtra, with 629 active infections. The Karnataka region showed a significant increase with 72 new cases, taking its active caseload to 395. On the other hand, there are no active Covid-19 cases in certain states like Arunachal Pradesh, Mizoram, and Manipur, hinting at effective containment measures. In spite of these diverse regional numbers, the overall national trend seems positively tentative. There were 1,420 new recoveries logged, elevating the total number of recovered or migrated persons to 10,976 for the year, which highlights India's resilience in its continuous Covid-19 response.
The increase is credited mainly to new Omicron sub-variants, such as JN.1, LF.7, NB.1.8.1, and XFG, which are highly contagious but produce milder symptoms than previous strains. These variants are now listed as "Variants Under Monitoring" by the World Health Organization (WHO). While not yet classified as variants of concern, they necessitate heightened vigilance, particularly among high-risk groups.
Experts advise against attributing these numbers to the onset of a full-blown wave. Rather, they refer to a seasonal surge, similar to the flu, which is consonant with the virus's post-pandemic shift towards an endemic pattern.
Why Are Booster Shots Not Recommended?
In light of India's high hybrid immunity from both vaccinations and past infections, health authorities as of now not recommending mass booster drives. Rather, targeted vaccination is being encouraged for:
- The elderly
- Immunocompromised hosts
- People with chronic respiratory or cardiovascular disease
The Indian Medical Association and health professionals emphasize that this risk-based, strategic strategy is more effective protection than mass immunization campaigns.
Even with the recent upsurge, health workers urge not panicking but remaining proactive. Distinguishing between Covid-19 and other common seasonal viral infections—often producing the same symptoms as fever, fatigue, and cough—is now of paramount importance.
Physicians and public health specialist, point out that exhaustion from sustained lookout can precipitate complacency. We can't ignore Covid-19 as a historical moment. It's very much still around—but controlled with the appropriate preventive measures.
Actionable Tips To Prevent COVID Infection
Nowadays, personal responsibility and daily routine are still your best protection. Here's how to protect yourself:
1. Wear a Mask in Crowded Indoor Spaces
Though mandates may have disappeared, masks are effective, especially in poorly ventilated spaces indoors, on public transportation, and in medical offices.
2. Get Current with Targeted Vaccines
If you are in a high-risk group, speak with your health care provider about boosters that are available or trials soon to be under way for new variant-specific vaccines.
3. Boost Your Immune System
Prioritize a balanced diet, proper sleep, and regular physical activity in order to maintain your immune system strong against not only Covid-19 but also other flu and seasonal diseases.
4. Practice Hygiene
Good hygiene habits such as regular handwashing, the use of sanitizers, and not touching your face while in public can significantly minimize the risk of transmission.
5. Stay Away from Crowds
If you are symptomatic—or have someone living with you who is—skip gatherings and go remote work whenever possible.
6. Identify Symptoms Early
Notice signs such as fever, chronic cough, body pain, fatigue, and taste/smell loss. Test early and self-isolate to avoid transmission.
7. Seek Medical Help
Particularly if above 60 years or with underlying conditions, don't wait even for minor symptoms. Early treatment can be life-saving.
The India Covid-19 situation is serious but under control. Yes, the active cases are more than 7,100, but the recovery rates are robust, and the fatalities are few and limited to the vulnerable groups.
Instead of fear, this time it requires a recommitment to public health practices—from mask-wearing when necessary to symptom-watching and protecting the vulnerable.
Key Figures at a Glance (as of June 13, 2025)
Total Active Cases: 7,131
New Cases in 24 Hours: 33
Total Deaths (2025): 78
Top States by Active Cases: Kerala (2055), Gujarat (1358), West Bengal (747), Delhi (714), Maharashtra (629)
Recovered in 2025: 10,976 and rising
Death IN Last 24 Hours: 1 (Kerala)
New Once-a-Week Pill May Transform Schizophrenia Treatment, Lancet Study Finds
Credits: Melanie Gonick/MIT
A team of researchers from MIT, New York Medical College, and Lyndra Therapeutics have introduced a once-a-week oral pill for schizophrenia. Published in The Lancet Psychiatry, this new treatment model could transform how antipsychotic medications are administered—enhancing patient compliance, improving long-term outcomes, and reducing relapse risk.
One of the most formidable challenges in treating schizophrenia is ensuring patients consistently take their medication. Daily dosing can be a significant hurdle, particularly in psychiatric conditions where cognitive and emotional symptoms hinder memory and motivation.
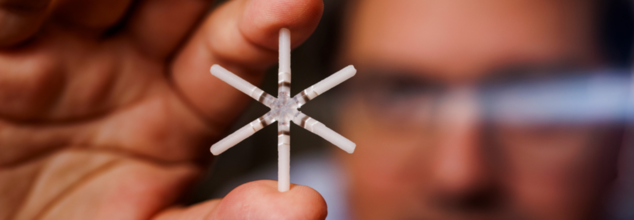
Enter the newly designed pill: a once-a-week oral capsule featuring a star-shaped drug delivery system that slowly releases risperidone over seven days. The innovation, led by Giovanni Traverso of MIT and Leslie Citrome of New York Medical College, aims to simplify schizophrenia treatment and drastically improve medication adherence.
"We’ve converted something that had to be taken once a day into a weekly oral solution using a novel delayed-release technology," said Traverso, also a gastroenterologist at Brigham and Women’s Hospital. “This innovation can be adapted for a variety of medications."
How Does The Star-Shaped Drug Delivery Device Work?
Roughly the size of a multivitamin, the capsule houses a six-armed, foldable device that expands once it reaches the stomach. This unique shape keeps the device in the stomach for about a week, allowing the steady release of medication.
Over time, each arm breaks off and safely passes through the digestive tract. This ensures the body gradually absorbs the medication while preventing gastrointestinal blockages. “It’s a breakthrough that allows drug delivery in a controlled and sustained manner,” said Dr. Citrome.
Promising Phase III Results
In the Phase III trial, 83 participants with schizophrenia were enrolled across five clinical sites in the US. Of these, 45 patients completed the full five-week regimen, taking one risperidone-loaded capsule per week.
The results were compelling. Drug levels peaked immediately post-dose and tapered slowly over the week. The consistency was superior to daily oral dosing, where patient-administered pills often result in fluctuating drug concentrations.
Symptom control, measured using the Positive and Negative Syndrome Scale (PANSS), remained stable across the board. Patients did not experience the spikes and troughs commonly observed with daily oral intake, a factor often linked to symptom relapses and hospitalizations.
Does This This Offer Minimal Side Effects With Major Potential?
The trial noted only mild side effects such as acid reflux and constipation, which were temporary and manageable. Importantly, the pill demonstrated an impressive safety profile over the trial duration.
“This really validates our hypothesis from over a decade ago—that a once-weekly oral drug delivery capsule could function as a depot system in the gastrointestinal tract,” Traverso remarked.
What are the Other Application?
Beyond schizophrenia, the research team is already eyeing broader applications for this delivery system. Phase I trials are being planned for drugs used in managing conditions like hypertension, asthma, and even for oral contraceptives.
The capsule’s ease of use, combined with its potential to deliver various drugs, makes it an attractive option for chronic conditions where daily adherence is challenging. Patients often prefer oral medications over injections, and this technology could serve as a bridge between convenience and efficacy.
Schizophrenia affects approximately 24 million people worldwide, according to the WHO. Medication adherence remains one of the key challenges in managing the condition. The consequences of missed doses can be severe—leading to relapse, hospitalization, or deterioration in quality of life.
The once-a-week oral capsule has the potential to fill this long-standing gap, offering hope for better disease control and reduced burden on caregivers and healthcare systems.
Dr. Richard Scranton, chief medical officer of Lyndra Therapeutics and senior author of the study, emphasized that the results support moving forward toward FDA approvals. “We’re incredibly optimistic about what this means for the future of psychiatric and chronic disease management.”
Lyndra Therapeutics and MIT are currently preparing for larger Phase III studies to confirm safety and efficacy on a broader scale. If successful, the capsule could be submitted for FDA approval within the next few years.
“This marks a paradigm shift in how we think about oral drug delivery,” added Robert Langer, MIT’s renowned bioengineer and co-founder of Lyndra. “And we’re just getting started.”
© 2024 Bennett, Coleman & Company Limited

