- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
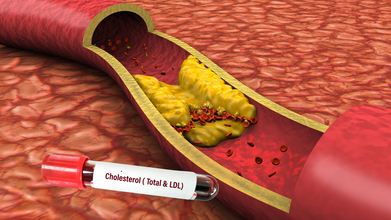
Breakthrough: Gene Editing Jab Dramatically Lowers Harmful Cholesterol Levels In First Trial
Credits: Canva
It may sound unreal, but by just altering one gene could one day help many people to permanently lower their dangerously high cholesterol and blood fat levels. This is the biggest takeaway from the latest research which had been unveiled at the American Heart Association's annual meeting in New Orleans.
The early stage human trial tested a first of its kind CRISPR based therapy, and the results were way better and way more powerful than what the cardiologists had expected.
What Is This Experimental Treatment All About?
The Phase 1 trial involved 15 adults, all with severely high levels of LDL cholesterol, or the bad cholesterol. They also had high triglycerides, some had both. The main goal of the experiment was not to prove whether the drug would improve health, but to confirm if it is safe enough to be tested in humans.
Dr Steven Nissen of the Cleveland Clinic, one of the study investigators, admitted he never imagined such a treatment would be possible. “The results were pretty spectacular,” he said.
Researchers say it passed that initial test. Even more striking, a single infusion of the gene editing therapy reduced LDL (the “bad” cholesterol) and triglycerides by around 50 percent. That kind of drop could sharply reduce a patient’s lifetime risk of heart attack and stroke.
How Does Gene Editing Work?
This treatment uses a gene editing tool called CRISPR. This tool can cut and alter DNA. This is thus able to target a single liver gene called ANGPTL#. While usually this gene boosts cholesterol levels by blocking the liver's ability to break down fats, some people may have naturally versions of this gene that do not work as strongly, and thus give them low cholesterol levels their entire lives.
The experimental drug is designed to mimic that natural advantage by turning off the gene permanently. Unlike statins, which must be taken daily, this therapy aims to work after just one dose. The drug, CTX310, was developed by CRISPR Therapeutics, which also funded the trial.
Excitement, But With Caution
The findings, which were simultaneously published in The New England Journal of Medicine, sparked a mix of enthusiasm and hesitation among heart experts.
Dr Karol Watson of UCLA called it an important proof of concept, but emphasized that permanent gene changes require long term safety data. “We already have safe, effective medications,” she said. “CRISPR must prove it’s both effective and safe over the long haul.”
Others note that many patients stop taking statins due to side effects, meaning a one time alternative could eventually fill a real need. Still, doctors stress that such a therapy must be studied for years before it becomes a mainstream option.
How Was The Study Conducted?
Most participants in the study were in their 50s or 60s, and lived in Australia, New Zealand, and in the UK. They received a single IV infusion lasting up to four and four and a half hours. Side effects were mild and temporary: nausea, back pain, and one brief increase in liver enzymes. One participant died months later from an unrelated cause.
Those who received the highest dose saw the biggest change. LDL levels fell by nearly 49 percent and triglycerides dropped by about 55 percent within two months.
Experts like Dr Elizabeth McNally of Northwestern say the dual effect on both LDL and triglycerides is promising, especially for people with very high triglycerides. Still, she cautioned that researchers must prove the therapy offers clear advantages over existing treatments.
Sally Kirkland Enters Hospice After Enduring 'A Challenging Few Months'

Credits: Wikimedia Commons
Sally Kirkland, American actress and producer has now entered hospice care in Palm Springs. The news was confirmed on November 9 by her rep Michael Greene, as per TMZ. The same was also updated on her GoFundMe page.
The November 7 post read from the account's manager read: "Thank you for all your love and support. Sally is grateful for your kindness and love. Sally is on hospice now and is resting comfortably. Please hold and send the light for Sally."
This update has come just over a month after organizers shared that Sally, who is now 84, had endured "a challenging few months".
What Happened To Sally?
On October 2, an update read: "Hi: Sally sends her love to everyone. It has been a challenging few months for Sally as her health continues to struggle. She had a fall in the shower, when she was left unattended, injuring her ribs and foot, along with cuts and bruises. Sally is now receiving 24/7 care in a specialized facility that is providing wonderful safety and care. We are continuing to raise money to cover the gaps between income and care costs. Thank you for all the love, support, and care for Sally."
Sally's fundraiser was created in November 2024, after Sally "fractured her four bones in neck, right wrist, and her left hip" and while she was recovering, she had developed "two separate life-threatening infections". Her medical bills would mount more than her insurance could cover.
She also created a video shortly after her GoFundMe account was made where she thanked everyone who donated. "Everyone, I just wanted to thank you for sending me your love and your light and helping me get through this, day by day, helping me with my GoFundMe page. It really touches my heart. [I] really feel your love."
What Is A Hospice?
Hospice is a specialized care focused on the comfort and quality of life for people with serious illnesses near the end of life. This happens typically when a cure is not possible. It provides physical, emotional, and spiritual support for the patient, as well as for their family. The hospice also aims to manage pain and symptoms, rather than focusing on life-prolonging treatments. This care can be provided at home, in a hospice center, a hospital, or a nursing home.
Why Do People Choose Hospice?
One of the key reasons why patients who have a degenerative condition choose hospice is because of the patient-centric care that it provides. A hospice has a team of professionals, including doctors, nurses, social workers, and chaplains, who provide a range of support to the patient and their loved ones. The care too is personalized to meet the needs of all individual patients, their needs, values, and preferences. The primary goal is to control their pain and other symptoms in order to maximize the patient's comfort and quality of life. There are also family-centered hospice that extends support to the family and loved ones of the patients, and help them cope with the illness and grief.
Hospice is typically used when a person has a prognosis of six months or less and has decided to stop treatment to cure or control their illness.
ByHeart Baby Formula Recalled As 10 US States Report 13 Cases Of Infant Botulism After Use

Credits: Canva
The Centers for Disease Control and Prevention (CDC), US, has issued a statement recalling an organic baby formula. The company ByHeart Inc's two lots of Whole Nutrition Infant Formula has been recalled, the lots are:
- Lot: 206VABP/251261P2 ("Use by 01 Dec 2026")
- Lot: 206VABP/251131P2 ("Use by 01 Dec 2026")
In a statement, the CDC wrote:
CDC and public health officials in several states, the Infant Botulism Treatment and Prevention Program, and FDA are investigating a multistate outbreak of infant botulism linked to recalled infant formula. Infant botulism happens when swallowed spores from a type of bacteria called Clostridium botulinum infects a baby's large intestine and make toxin in it. Infant botulism often starts with constipation but is usually first noticed as difficulty feeding (sucking and swallowing), a weak and altered cry, and loss of muscle tone.
The CDC also notes that 10 states have seen the cases infant botulism. 13 cases have been reported, all of them leading to hospitalization.
The Food and Drug Administration (DA), US has also asked parents and caregivers who have this product to identify the given lot information at the bottom of the packaging and if it matches, they must throw it away. The FDA has said that it is working with retailers to remove "all potentially impacted product" from the store shelves.
FDA has also asked parents who have fed their kids ByHeart's infant formula to keep and eye on them as a precaution and botulism can take two weeks to develop.
The States Which Have Reported The Cases Of Infant Botulism Linked With ByHeart Formula:
- Arizona
- California
- Illinois
- Minnesota
- New Jersey
- Oregon
- Pennsylvania
- Rhode Island
- Texas
- Washington
What Is Infant Botulism?
Most common form of all botulism in babies, who are between 2 to 8 months old. It happens when the bacteria spores grow in a baby’s intestines and produce the toxin. Honey and contaminated soil can be sources of infant botulism. Adults can also get this type, though it’s rare.
What Are The Symptoms Of Botulism In Infants?
As per CDC, the symptoms include:
- Most infants with infant botulism will initially develop constipation, poor feeding, loss of head control, and difficulty swallowing
- If untreated, infants with infant botulism experience a progressive flaccid paralysis that can lead to breathing difficulties and required weeks of hospitalization
The CDC has also recommended that if clinical supports infant botulism then parents and caregivers must begin the treatment and should not wait for laboratory confirmation.
What Did The Makers Of The Infant Formula Say?
ByHeart, which is a New York City based company said that the FDA has as of now tracked 83 reports of infant botulism across the nation since August, 13 of them from the formula. In the statement, the company said: "ByHeart is taking the proactive step to remove any potential risk from the market and ensure the highest level of safety for infants. The FDA has not identified a direct link between any infant formula and these cases and there is no historical precedent of infant formula causing infant botulism."
Mounjaro Becomes India’s Top-Selling Medicine in October; What’s Driving The Surge

Credits: Canva
Eli Lilly’s weight-loss and diabetes injection, Mounjaro, has overtaken GSK’s antibiotic Augmentin (GSK.L) to become India’s highest-selling drug by value in October, as demand for weight-loss treatments continues to surge in the world’s most populated country. According to new data from research firm Pharmarack, the U.S.-based drugmaker’s popular injectable earned ₹1 billion ($11.38 million) in sales last month, compared to Augmentin’s ₹800 million.
While Augmentin still led in overall units sold—5.8 million doses versus 85,000 of Mounjaro—the latter’s premium pricing pushed it ahead in total value, as per Reuters. Analysts say India is rapidly emerging as a major market for obesity treatments, with the global weight-loss drug sector expected to cross $150 billion annually by the end of this decade.
Eli Lilly’s Mounjaro Becomes India’s Top-Selling Medicine in October
Launched in India in March 2025, Mounjaro which helps regulate blood sugar levels and reduces appetite—has seen its sales double within months, surpassing its rival Wegovy by Novo Nordisk, which entered the Indian market in June. Data from Pharmarack shows that Mounjaro has so far generated ₹3.33 billion in total revenue.
“Mounjaro’s consumption in India by volume was nearly ten times higher than Wegovy in October,” said Sheetal Sapale, Vice President (Commercial) at Pharmarack. Eli Lilly sold around 262,000 doses of Mounjaro last month, compared to 26,000 doses of Wegovy. Both drugs belong to the GLP-1 receptor agonist class, designed to treat obesity and type 2 diabetes.
How Does Mounjaro Work?
Mounjaro is a once-weekly injectable medication developed primarily for managing type 2 diabetes. Its key ingredient, tirzepatide, is the first of its kind to act on two gut hormones—GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide). This dual mechanism makes Mounjaro more advanced than older drugs such as Ozempic (semaglutide) or Victoza (liraglutide), which target only GLP-1, which is thus driving a surge in its sale in india.
Although it was originally approved for diabetes management, Mounjaro has attracted global attention for its strong weight-loss effects. Ongoing studies are evaluating its use for non-diabetic individuals struggling with obesity. The drug mimics the action of GIP and GLP-1, which are hormones released naturally after meals to regulate insulin release, lower blood sugar, and suppress appetite.
Mounjaro for Type 2 Diabetes
For people living with type 2 diabetes, keeping blood sugar in check often requires a combination of medicines, diet adjustments, and regular monitoring. Mounjaro offers a more integrated solution by targeting multiple factors that influence glucose levels. Findings from clinical trials, including the SURPASS-1 to SURPASS-5 studies, show that Mounjaro delivers better outcomes than many existing diabetes treatments, particularly in lowering HbA1c levels—a key marker of long-term glucose control.
Patients using Mounjaro have shown:
- Lower fasting and post-meal blood sugar levels
- Reductions in HbA1c by up to 2.5%
- Improved insulin sensitivity
Mounjaro and Weight Loss
Beyond its use for diabetes, Mounjaro has gained global popularity for its significant role in weight reduction. Clinical studies reveal that people taking the drug often lose between 15% and 20% of their body weight over several months of consistent use. This level of weight loss surpasses that seen with many earlier treatments and even some surgical interventions, making Mounjaro one of the most effective options currently available for managing obesity.
Disclaimer: This article is intended for general information and should not replace professional medical guidance. Always consult your doctor for advice or concerns regarding any health condition.
© 2024 Bennett, Coleman & Company Limited

