- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Explained: After UK, Scotland Too Bans Puberty Blockers For Under 18, Know What It Means

Credits: Canva
Recently on December 11, the British government indefinitely banned puberty blockers for children with gender dysphoria. This step was taken after independent experts found that there was an unacceptable safety risk involved when prescribing this medication.
Before getting into the "why" of the ban, let's understand what gender dysphoria is and what puberty blockers do.
Gender Dysphoria
As per the National Health Service (NHS), UK, gender dysphoria is a term that describes a sense of unease that a person may have because of a mismatch between their biological sex and their gender identity. The NHS writes, "Most people identify as "male" or "female". These are sometimes called "binary" identities. However, some people feel their gender identity is different from their biological sex. For example, some people may have male genitals and facial hair but do not identify as a male or feel masculine."
NHS notes that children show their interest through the choice of toys and clothes that are often associated "societally" with the opposite gender.
Puberty Blocker
They are a type of medication that delays the changes of puberty in gender-diverse youth. They are called gonadotrophin-releasing hormone (GnRH).
How does it work?
Sex hormones get affected once you consume this medicine. Sex hormones determine the sexual organs present at birth, which are also known as the primary sex characteristics. They include the penis, scrotum and testicles, and the uterus, ovaries and vagina. In contrast, the secondary sex characters are the organs or physical changes that occur in our body after we hit puberty. For instance, facial hair, development of breasts, etc.
So what do puberty blockers do?
It delays puberty, which can combat the symptoms, as noted by NHS, which can occur if someone experiences gender dysphoria. The symptoms include:
- low self-esteem
- becoming withdrawn or socially isolated
- depression or anxiety
- taking unnecessary risks
- neglecting themselves
The puberty blocker by delaying the development of secondary sex character helps with the:
- improving mental well-being
- ease depression and anxiety
- improve social interactions with others
- lower the need for future surgeries
- ease thoughts or actions of self-harm
So, why is it banned?
The UK stated that this decision will be revisited in 2027, till then the ban remains effective. However, it goes against standards held by medical groups including the European and World Professional Associations for Transgender Health and American Medical Association and the American Academy of Pediatrics.
The ban was put in place by the Conservative government and has been extended by the Labour government too. The announcement comes after a judge upheld an emergency ban in a ruling that said this treatment could be potentially harmful. However, NHS also stopped prescribing puberty blockers at gender identity clinics stating that there was not enough evidence found for either benefits or harms.
However, in July, Justice Beverley Lang said that the review commissioned by the NHS found "very substantial risks and very narrow benefits” to the treatment. The confusion exists because the British Medical Association further noted that the NHS review was controversial and included patients, academics, scientists and legal experts among its critics. However, the group that challenged the court - TransActual criticized the decision and said that evidence of danger from 40 years of puberty blocker cannot be tracked.
Banning medicines with no evidence of serious harm, only for trans people … is discrimination plain and simple,” said Keyne Walker, the group’s strategy director. “Evidence of the harm of the temporary ban continues to emerge, and will grow now that it has been made permanent.”
Why the ban in Scotland?
The Scottish government also confirmed that as the medicine policy is reserved to Westminster, the ban would also apply across England, Scotland and Wales.
However, the ban is not on those who are already consuming the medicine for gender dysphoria, but on the "new children and young people aged under 18 years from beginning to take puberty blockers for the purposes of gender incongruence and/or gender dysphoria. under the care of private or non-UK prescribers".
Passive Euthanasia: Harish Rana’s Case May Reshape End-of-life Protocols, Say Experts
Credit: iStock
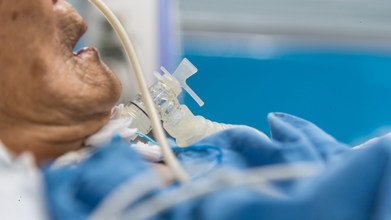
The Supreme Court of India, in a landmark decision, authorized the removal of life support for Harish Rana, a 31-year-old man in a vegetative state since 2013.
This marks the country's first Court-approved case of passive euthanasia without a prior living will. The Court ruled that the "right to die with dignity" is a fundamental part of the right to life under Article 21.
Also read: Supreme Court Allows 1st Passive Euthanasia For Man In Vegetative State For 13 Years
Speaking to HealthandMe, the experts said that the landmark ruling will enable families and doctors to make compassionate decisions and may also influence end-of-life protocols.
There are several medical conditions where patients undergo prolonged suffering despite treatment, with no realistic scope for recovery, sometimes for decades.
“This judgment could have a significant impact on end-of-life care practices in Indian ICUs. Many patients remain in prolonged vegetative states with no meaningful quality of life, often sustained only through artificial life support,” Dr. Sandeep Dewan, Senior Director, Critical Care & Chairman ECMO Program, Fortis Gurugram, told this publication.
“The ruling reinforces that while preserving life is important, the quality and dignity of life must also be considered, and it provides clearer pathways for families and doctors to make compassionate decisions in such situations,” he added.
Harish was a BTech student in Chandigarh who suffered severe traumatic brain injury after falling from the fourth floor of his paying guest accommodation in August 2013.
Since then, he has remained bedridden and was being treated with Clinically Administered Nutrition (CAN), where surgically installed PEG tubes helped him with breathing and nutrition.
The apex Court, in its ruling, noted that it can just prolong his biological existence, but it will not lead to any therapeutic improvement.
With the Harish Rana judgment, the apex Court today clarified how passive euthanasia should be applied in cases where a patient’s life is being supported by feeding tubes.
The top Court also waived off the reconsideration period of 30 days and noted that the medical treatment, including the CAN administered to the patient, can be withdrawn or withheld.
"Doctors and hospitals have often been reluctant to stop tube feeding in such patients, fearing that it could be interpreted as 'starving the patient to death',” Dr. Rajeev Jayadevan, Ex-President of IMA Cochin and Convener of the Research Cell, Kerala, told HealthandMe.
“Today’s ruling clarifies that artificial nutrition and hydration are indeed forms of medical treatment. Therefore, withholding such artificial feeding can be considered withdrawal of life-sustaining medical support in situations where treatment offers no prospect of recovery and only prolongs suffering,” he added.
Passive Euthanasia In India
Passive Euthanasia allows a terminally ill or irreversibly comatose patient to die naturally. It involves deliberately withholding or withdrawing life-sustaining treatments (like ventilators, feeding tubes, or medication). It has been legal since 2018, but under strict guidelines.
On the other hand, active euthanasia or assisted suicide for terminally ill patients is legal in several countries, but is not permitted in India.
The Aruna Shanbaug Case (2011) paved the way for passive euthanasia in India.
Shanbaug was a nurse at Mumbai's KEM hospital who remained in a vegetative state for 42 years after an assault in 1973. The hospital staff cared for her and did not stop treatment till she passed away naturally in 2015.
However, in the 2011 Aruna Shanbaug judgment, the SC allowed passive euthanasia by permitting the withdrawal or withholding of life-sustaining treatment under strict legal safeguards.
This framework was further clarified in the 2018 Common Cause judgment, which recognized advance directives or living wills.
Later in 2023, the SC modified the guidelines, noting that withdrawal of life support is permissible only after the approval of the Primary and Secondary Medical Boards.
A Living Will
Dr. Jayadevan noted that, as death is a certainty for all who are living, greater awareness must be created on adults preparing a "Living Will or Advanced Directive".
A Living Will is essentially made when individuals are "still in good health— documenting one’s preference for specific treatment measures in the event of a terminal illness occurring in the future”.
“This will help relatives and doctors to take the right decisions and avoid unnecessary treatment measures in such situations. Unlike the conventional Will that is executed after death, a Living Will is implemented when a person is still alive,” the doctor said.
Supreme Court Allows 1st Passive Euthanasia For Man In Vegetative State For 13 Years

Credit: iStock
In a landmark judgement, the Supreme Court today allowed passive euthanasia for a 32-year-old man, living in a vegetative state for the last 13 years.
A bench comprising Justice JB Pardiwala and Justice KV Viswanathan allowed the withdrawal of life support for Harish Rana, a resident of Ghaziabad, who has been in a coma and kept alive on tubes for breathing and nutrition after sustaining severe head injuries following a fall from a building in 2013 in Chandigarh.
It is the first known case of a court-ordered passive euthanasia in India, since it was legalised in 2018 and modified in 2023, recognizing the fundamental right to die with dignity.
"Harish Rana, presently aged 32 years, was once a young, bright boy. He met with a tragic life-altering accident after a fall from the fourth floor of his paying guest accommodation. His brain injury left him in a condition of Persistent Vegetative State (PSV) with 100 percent quadraplegia... Medical reports show that his medical condition has not improved in the past 13 years," LiveLaw quoted the bench as saying.
The Court noted that the continuation of his treatment -- Clinically Administered Nutrition (CAN) via surgically installed PEG tubes -- can just prolong his biological existence but will not lead to any therapeutic improvement.
What Is The Case Of Harish Rana?
Harish was a BTech student in Chandigarh who suffered severe traumatic brain injury after falling from the fourth floor of his paying guest accommodation in August 2013.
Since then, he has remained bedridden and dependent on others for all activities of daily life.
Harish's father, the petitioner, first approached the Delhi High Court in 2024, seeking permission for passive euthanasia, but was rejected as the patient was not terminally ill.
The same year, the petitioner knocked on the doors of the Supreme Court, which, though it refused to entertain the plea, directed the Uttar Pradesh government to bear the treatment expenses.
In 2025, the petitioner filed a miscellaneous application in the Supreme Court, noting that Harish's condition had no scope for improvement.
The Court then directed the constitution of a Primary Medical Board led by the District Hospital in Noida to examine his health, as well as a Secondary Medical Board constituted by the All India Institute of Medical Sciences (AIIMS).
After perusing the report, Justice Pardiwala remarked that it's a "sad report" and the man can't continue to live like this. Before passing the final order, the Court met the parents, LiveLaw reported.
The Court has asked AIIMS to provide palliative care, so that the withdrawal of CAN can be given effect to.
To maintain the dignity of death, the apex Court said that the life support must be withdrawn with a tailored plan.
1st Passive Euthanasia: What's New From The 2018 Judgment
In 2018, a five-judge Constitution Bench had recognized and given sanction for passive euthanasia, and living will/advance directives.
Later in 2023, the SC modified the guidelines, noting that withdrawal of life support is permissible only after the approval of the Primary and Secondary Medical Boards.
With the Harish Rana judgment, the apex Court today clarified how passive euthanasia should be applied in cases where a patient’s life is being supported by feeding tubes.
The top Court waived off the reconsideration period of 30 days and noted that the medical treatment, including the CAN administered to the patient, can be withdrawn or withheld.
What is Passive Euthanasia
Passive Euthanasia allows a terminally ill or irreversibly comatose patient to die naturally. It involves deliberately withholding or withdrawing life-sustaining treatments (like ventilators, feeding tubes, or medication). It has been legal since 2018, but under strict guidelines.
In Active Euthanasia, patients are administered a lethal injection to cause death. It is illegal in India and considered an offence.
The Aruna Shanbaug case in 2011 opened the door for passive euthanasia in India for the first time.
The top Court rejected euthanasia in the case of Shanbaug, a nurse at Mumbai's KEM hospital who was in a vegetative state for 42 years after an assault in 1973, as the hospital staff who cared for her for decades did not support stopping treatment.
Shanbaug continued to be under care and passed away naturally in 2015
However, in her case, the court made the judgment allowing for passive euthanasia in certain rare situations under strict conditions.
Doctors Call For Stricter Rules to Curb Risks In Hair Transplant, Cosmetic Treatments

Credit: iStock
In the wake of a shocking incident in Uttar Pradesh’s Kanpur, where two engineers allegedly died within 48 hours of undergoing hair transplant surgery by a dentist, the Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) and the Association of Plastic Surgeons of India (APSI) have pressed the need for stricter rules for aesthetic and hair restoration procedures.
The doctors raised concerns about patient safety and called for ramping up training standards, even as many such cases where unqualified medical practitioners performed aesthetic procedures leading to severe infections, loss of sight, and many complications have been documented from across the country.
Traditionally, these procedures were performed by specialists such as dermatologists and plastic surgeons trained under the regulatory framework of the National Medical Commission (NMC).
However, experts said the issue has become more complicated after the Dental Council of India (DCI) allowed MDS dental surgeons, under provisions of the Dentists Act, 1948, to perform certain aesthetic procedures and hair transplantation.
“Aesthetic procedures and dermatology demand additional training. In addition to the MBBS degree, a dermatologist training program requires three years of residency at a postgraduate level in dermatology at certain accredited medical schools,” Dr Vinay Singh, President IADVL said.
He added that the training also includes a condensed curriculum of various skin ailments, hair problems, and advanced procedures in dermatology.
“Allowing professionals without comprehensive medical training in skin diseases, hair disorders, and surgical complication management to perform such procedures could dilute training standards and increase risks for patients,” warned Dr. Rajat Gupta, Senior Consultant Plastic Surgeon, Delhi.
The experts also pointed out that hair transplant is a modern medical procedure and should only be conducted by Registered Medical Practitioners (RMPs) who are specialized in that area.
Also read: Fact Check: Popular Hair Loss Treatment Ingredient Could Trigger Chest Pain
Dr. Aditya Aggarwal, Senior Consultant Plastic Surgery, Medicity Medanta Hospital, shared that the surgery requires knowledge regarding the biology of the skin, the disorders of the hair, how to manage infections, and how to manage complications.
The associations urged the government to issue comprehensive guidelines and ensure strict implementation of existing regulations to curb quackery and safeguard public health.
Further, they advised the patients to verify the doctor’s qualifications and registration with the state medical council before undergoing any skin, hair, or cosmetic treatment.
The public must remain alert and avoid falling prey to misleading advertisements or treatments offered by unlicensed practitioners, the experts said.
© 2024 Bennett, Coleman & Company Limited

