- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
FDA Approves ZYN Nicotine Pouches As Safer Alternative For Adult Tobacco Users

The U.S. Food and Drug Administration (FDA) has taken a significant step in the evolving landscape of tobacco harm reduction by authorizing the marketing of 20 ZYN nicotine pouch products. The agency released this decision following comprehensive scientific review under the PMTA pathway, marking the first time FDA has authorized nicotine pouches. This move, while bringing to light debates concerning public health, could fundamentally shape the future of nicotine consumption and alternatives to tobacco use in the United States.
ZYN nicotine pouches are innovative products designed as alternatives to traditional tobacco. Unlike cigarettes or smokeless tobacco, these small synthetic fiber pouches contain nicotine but no tobacco. Users place them between the gum and lip, where nicotine is absorbed into the bloodstream. Available in various flavors, including cinnamon, citrus, and cool mint, ZYN offers two nicotine strengths- 3 milligrams and 6 milligrams.
This new product category targets adult smokers who would like to switch to less harmful alternatives compared to traditional tobacco. FDA's authorization marks a landmark moment for this emerging segment of the tobacco market in the United States.
Why Did the FDA Approve ZYN?
The FDA decision anchors from the Family Smoking Prevention and Tobacco Control Act of 2009, which obligates all new tobacco products to comply with strong public health standards. The agency review found that the ZYN pouches involve substantially lower risks compared to cigarettes and smokeless tobacco products, such as snuff or snus.
The pouches contain far fewer harmful constituents associated with cancer and other life-threatening diseases. In addition, studies submitted by the manufacturer showed that many adult smokers and users of smokeless tobacco switched entirely to ZYN, indicating its potential as a harm-reduction tool.
"These nicotine pouch products meet that bar by benefiting adults who use cigarettes or smokeless tobacco products," said Matthew Farrelly, Ph.D., director of the FDA's Office of Science at the Center for Tobacco Products.
How This Will Help Manage Youth Tobacco Use?
This is a clear FDA warning: ZYN is not risk-free, nor is it "FDA-approved." Although the agency said that low use levels of nicotine pouches among youths remain at 1.8% of middle and high school students in the United States who reported using any tobacco product, according to the 2024 National Youth Tobacco Survey, the agency emphasized preventing youth exposure.
The FDA did this by applying stringent marketing restrictions, including the following:
- Ban mass-market advertising on TV and radio.
- Require actors/models in ads to appear at least 35 years old.
- Avoid themes, characters, or imagery appealing to youth.
The agency will also closely monitor marketing practices and suspend or withdraw authorizations if products no longer meet public health standards.
ZYN pouches don't contain any tar and the carcinogenic chemicals present in cigarette smoke and in the majority of smokeless tobacco products. Unlike snus, a popular form of pasteurized tobacco widely used in Scandinavian countries, ZYN contains no tobacco at all. In fact, that positions it like the nicotine gum or lozenge, releasing controlled doses of nicotine to eliminate craving.
For decades, tobacco companies have been looking for alternatives to traditional cigarettes, as smoking rates in the U.S. and around the world have declined. E-cigarettes became popular in the early 2010s but were later criticized for underage vaping. ZYN nicotine pouches could be a safer alternative, but critics fear they may follow the same path if youth use increases.
ZYN, marketed by Philip Morris International acquisition target Swedish Match through 2022 for a cost of $16 billion. While Philip Morris invests massively into nicotine pouches, huge demand from customers bolsters the business segment in the most phenomenal way in U.S. tobacco. Already nicotine pouches emerge as a pace-setter that could set to be at the core of industry's future prospects.
Also Read: 3 Science-Backed Methods To Quit Smoking For Good
However, controversies run rampant in the history of tobacco alternatives. For example, e-cigarette giant Juul Labs was sued for marketing to minors, settling in 2023 for $462 million without admitting wrongdoing. Critics are now beginning to scrutinize ZYN flavored products, including citrus and peppermint, arguing they may attract younger users.
That benefit, however – the FDA seems poised to acknowledge adult smokers will use nicotine pouches – underscores much-needed responsible marketing and rigorous oversight. Public health advocates caution, however, there is no such thing as winning when it has to be over youth uptake first and close to long-term effects on health.
Supporters say that ZYN is a genuine substitute for smokers looking to quit or reduce their reliance on devastating tobacco products. Indeed, as Brian King, Ph.D., FDA's Center for Tobacco Products director, noted, "It is imperative that the maker of these products does so responsibly so that youth will not take up their use".
The FDA's approval of ZYN nicotine pouches is part of a larger effort to regulate and innovate within the tobacco industry. Over the past decade, the agency has received applications for nearly 27 million products, authorizing only those that meet rigorous health standards.
This also goes with recent suggestions to limit nicotine content in cigarettes, marking a step toward harm reduction and prioritization of public health. However, challenges remain, but the ZYN authorization is reflective of a growing recognition of alternative nicotine products' role in reducing tobacco-related disease and mortality.
The FDA's approval of ZYN nicotine pouches marks a turning point in tobacco harm reduction. As a less harmful alternative to cigarettes and smokeless tobacco, ZYN has the potential to benefit adult smokers while reducing risks to public health. However, the success of this initiative will depend on strict regulatory oversight, responsible marketing, and continued vigilance against youth use.
Effective Tips for Quitting Smoking
Smoking cessation is one of the best heart-healthy lifestyle choices you could ever make and minimize the risk for developing conditions like A-Fib. While quitting smoking might not be an easy task, its benefits can be felt at once and significantly. Here is how to quit smoking successfully.
1. Decide on a quit date
Set a quit date and be firm about it. This will give you ample time to mentally and physically prepare for the transition.
2. Build a Support System
Share your decision with your family, friends, or support group. This will make it easier for you because you have people to motivate and hold you accountable.
3. Use Nicotine Replacement Therapy
Consider using nicotine patches, gum, or lozenges to manage withdrawal symptoms and reduce cravings. Seek the advice of a healthcare professional.
4. Identify and Avoid Triggers
Identify situations or emotions that make you want to smoke, such as stress, boredom, or social settings. Develop healthy coping strategies, like journaling or engaging in a hobby, to navigate these triggers.
5. Stay Active
Engage in regular exercise. Exercise not only diverts your mind from excessive hunger and craving but also improves moods and overall well-being.
6. Keep Hydrated
Drinking water will help to flush out nicotine from your system, and the level of your craving will decrease.
7. Stress Management
People often smoke when their stress is too high. Try to engage in some relaxing activities like yoga, meditation, or deep breathing.
Tobacco Product Use Among Middle and High School Students — National Youth Tobacco Survey, United States, 2024
E-Cigarette and Nicotine Pouch Use Among Middle and High School Students — United States, 2024
What Is Jess's Rule? How A Missed Cancer Diagnosis Led To A New Rule For General Practitioners

(Credit-Andrea Brady)
GPs in England are being urged to adopt a “Jess’s Rule”, an initiative that urges doctors to rethink their treatment if, even after three patient appointments, the patient is still experiencing the same undiagnosed symptoms. This new initiative, called Jess's Rule, is being rolled out across the NHS to help prevent avoidable deaths by catching serious illnesses earlier, especially in young people. The rule is not a law, but a strong directive designed to make a more thorough diagnostic process standard practice nationwide.
Who Is Jess from Jess’s Rule?
While this new rule may help many people, it is a direct result of the tragic story of Jessica Brady. At just 27, she passed away from advanced stage 4 cancer in 2020. Before her diagnosis, she had more than twenty appointments with her GP practice over five months, all while her symptoms were getting progressively worse. Despite her persistence, her condition was repeatedly misdiagnosed or dismissed.
Ultimately, her family had to seek private care, and only then was she diagnosed with a type of cancer so advanced that no treatment was possible. She died just three weeks after her diagnosis. Jessica's story highlights the critical need for a system that ensures a patient's concerns are taken seriously, especially when initial treatments or diagnoses aren't working.
Who Is At Risk For Less Cancer Diagnoses?
A big aspect of Jess's Rule is its focus on reducing health inequalities. Studies have shown that younger patients and those from minority backgrounds often face delays in getting a proper diagnosis. One of the reasons given was that their symptoms might not fit the typical patterns seen in older or white patients, which then leads to their concerns being dismissed.
According to Gov.UK, a report found that half of all 16 to 24-year-olds with cancer needed three or more appointments to get a diagnosis. Jess's Rule is made to combat this problem by focusing on the fact that a patient's age or background should not be a reason to overlook a potential serious illness.
The NHS hopes that this rule, which is urged to be followed across the nation, will help ensure everyone receives the same high standard of care. This initiative is a tribute to Jessica's legacy and her family's tireless efforts to prevent other families from enduring similar grief.
How Does Jess’s Rule Work?
Jess's Rule isn't a strict law, but it's a guide for medical professionals. When a patient's symptoms don't improve after three appointments, the rule suggests that the GP should take further action. This step might include moving from phone consultations to a face-to-face appointment, conducting a more in-depth physical examination, or ordering additional tests.
It also encourages doctors to review the patient's records carefully, consult with a colleague for a second opinion, or refer the patient to a specialist. The aim is to formalize what many good doctors already do: if something isn't right, don't give up.
According to Health and Social Care Secretary Wes Streeting, Jessica's death was a "preventable and unnecessary tragedy," and Jess's Rule will ensure that every patient receives "thorough, compassionate, and safe care." The initiative is a collaborative effort between the Royal College of General Practitioners (RCGP) and NHS England.
What Is Leucovorin? The Drug Trump Suggests As A Treatment For Autism?
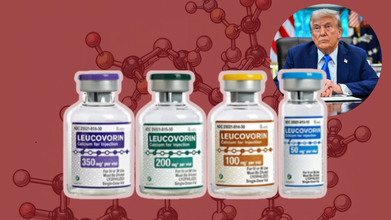
Credits: AP, TradeIndia
US President Donald Trump held a press conference to announce the connection between the use of Tylenol, a popular pain and fever relief medicine during pregnancy and autism. Trump in the press conference also suggested Leucovorin, as a potential treatment for autism symptom.
Read: Tylenol And Autism: Trump Announces Link Between The Two, Science Does Not Back Him
What Is Leucovorin?
It is known as folinic acid, a form of folate, also an essential B vitamin or B9, which has been approved by the US Food and Drug Administration (FDA) for counteracting the toxic effects of certain cancer drugs. It can counteract methotrexate that blocks body's use of folate.
The drug is also used to enhance the effects of chemotherapy drugs and to treat specific types of anemia.
How Is Leucovorin Administered? Who Makes Leucovorin?
The drug can be administered orally or intravenously. While it is manufactured by dozens of companies, notes US FDA, on Monday itself, FDA published a notice to the Federal Register ahead of Trump's press conference to approve a version of Leucovorin manufactured by GlaxoSmithKline or GSK.
GSK had previously withdrawn from FDA's consideration when it stopped making the drug. However, as per the FDA notice, "The FDA is working with GSK, the innovator of Wellcovorin (leucovorin calcium), on a process to include the essential scientific information needed for the safe and effective use of these drug products for adults and pediatric patients with CFD (cerebral folate deficiency)."
Is Leucovorin Effective For Autism?
As per the FDA notice, leucovorin calcium, branded as Wellcovorin by GSK is sent for approval for patients with cerebral folate deficiency, which is a neurological condition that affects folate, an essential vitamin for brain health, transport into the brain.
The FDA notice mentions that individuals with CFD have been "observed to have developmental delays with autistic features".
FDA Commissioner Marty Makary, MF, MPH said that in over two decades, the US has "witnessed a tragic four-fold increase in autism". He further noted: "Children are suffering and deserve access to potential treatments that have shown promise. We are using gold standard science and common sense to deliver for the American people.”
George Tidmarsh, MD, PhD, Director of the FDA's Center for Drug Evaluation and Research said that FDA is collaborating with GSK to broaden the existing Wellcovorin label and is committed to "finding and treating the root causes of autism".
Read: Trump's Claim On Linking Tylenol And Autism Is Unscientific, According To Doctors
Why Leucovorin Is Considered To Treat Autism?
Doctors have been using leucovorin off-label for autism, repurposing the drug beyond its original approval, reported Reuters.
Research from SUNY Downstate Medical Center and others suggests that as many as three-quarters of children with autism may have genetic variations or autoimmune issues that interfere with folate processing in the brain.
Smaller studies have linked these problems to more severe autism symptoms and found that leucovorin treatment may help improve speech, social interaction, and irritability. Still, the Autism Science Foundation cautions that the research is in its early stages, and more evidence is needed before firm conclusions can be drawn.
Read: Trump Suggests Changes In Childhood Vaccines, Says It Is Based On What He Feels
A 2016 study published in Molecular Psychiatry, which was a randomized controlled trial suggested that this specialized form of folate can improve communication and language skills in some children with Autism Spectrum Disorder (ASD).
A June 2020 study published in the Seminars in Pediatric Neurology suggests folate (vitamin B9) plays a vital role in brain development and function, helping with DNA synthesis, methylation, and neurotransmitter production. Some children with autism struggle with folate metabolism, particularly in transporting folate into the brain, a condition known as cerebral folate deficiency.
Another 2020 study published in October in Seminars in Pediatric Neurology suggests that in many cases, the immune system produces antibodies that target the folate receptor alpha (FRα) on brain cells. These folate receptor autoantibodies (FRAA) block folate entry into the brain, resulting in low brain folate levels even when blood levels are normal. While only about 5–10% of the general population carries these antibodies, research suggests that 50–70% of children with autism may have them. This disruption is thought to significantly affect speech and cognitive development.
However, Dr. David Mandell, a professor of psychiatry and autism expert at the University of Pennsylvania, told Reuters that leucovorin might well be a possible treatment for some children with autism, "but the evidence we have supporting it... is really, really weak."
Trump's Claim On Linking Tylenol And Autism Is Unscientific, According To Doctors

Credits: AP and Kenvue
The Monday announcement by President Trump on Tylenol and its link with autism has sparked a debate among the medical field. Experts have claimed that the link suggested is unfound in science. Australia's peak body for obstetricians and gynaecologists now fear that pregnant women will not take paracetamol when they need it and suffer harm from unmanaged fever.
Trump suggested that women should "fight like hell" and not take paracetamol, which is branded as Tylenol in the US, as it heightens the risk of autism when it is used by pregnant women. He further said that if women continue to take the medicine, "that would mean you can't tough it out, so that's up to you and your doctor. I just want to say it is like it is: don't take Tylenol. Don't take it. Fight like hell not to take it."
However, as far as science and facts are concerned, the pain medicine is widely considered a safe option to treat pain and fever even during pregnancy.
As a result of this, Australia's medicine regulator, the Therapeutic Goods Administration (TGA), re-confirmed the safety of this drug use during pregnancy.
Also Read: Tylenol And Autism: Trump Announces Link Between The Two, Science Does Not Back Him
What Is The Australian Medical Body Saying About Tylenol Use?
The Royal Australian and New Zeeland College of Obstetricians and Gynaecologists (RANZCOG) warned that Trump’s comments could lead pregnant women to avoid paracetamol and suffer harm from unmanaged fever.
Dr Elisha Broom, a counsellor and spokesperson for RANZCOG, as reported by Guardian said, evidence supports the safety of paracetamol in pregnancy when used correctly. Broom further noted that unmanaged fever is a known risk to pregnancy, unlike paracetamol.
“It’s not a no-harm scenario when women are fearful to take what we know are safe medications,” said Broom.
Broom is a maternal fetal medicine sub-specialist in Queensland and said that many obstetricians are expecting questions from their patients after Trump's announcement and would welcome them.
Read: What Is Leucovorin - The Drug Trump Suggests As A Treatment For Autism?
Is There A Link Between Fever Medicines And Babies?
Broom said, "We know that actually there is a link between fever and impacts on babies – not neurodivergence – but complications in pregnancies that result from unmanaged fever."
She further noted that "It is not a no-harm scenario where women are fearful to take what we know are safe over-the-counter medications to relieve pain and fever in pregnancy."
The reason why this announcement could harm women more than protecting them is because medicines like Nurofen or ibuprofen is already not recommended because it does not have the same safety profile, suggests Broom. Thus, this leaves women without any options but to cope with pain and unmanaged fever.
The TGA also noted that "paracetamol remains Pregnancy Category A in Australia, meaning that it is considered safe for use in pregnancy”.
It said: “The TGA maintains robust post-market safety surveillance and pharmacovigilance processes for all medicines registered in Australia, including paracetamol.”
Read: Trump Suggests Changes In Childhood Vaccines, Says It Is Based On What He Feels
What Do Other Medical Boards Say About This Announcement?
Australian Medical Association (AMA) president, Danielle McMullen stressed that studies showing a “possible association” between paracetamol and autism do not prove causation. She said autism is far more likely to be driven by genetic factors.
The American College of Obstetricians and Gynecologists (ACOG) also called Trump's announcement "irresponsible" and "highly unsettling". They emphasized acetaminophen (Tylenol) remains the safest painkiller during pregnancy.
© 2024 Bennett, Coleman & Company Limited

