- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
FDA Escalates Recall Of 64,800 Lbs. of Butter Over Undeclared Allergen

Credits: Canva
FDA Recalls Butter: In a growing food safety alert, the U.S. Food and Drug Administration (FDA) has escalated a butter recall to a Class II risk level following concerns over undeclared allergens. The product in question, European Style Butter Blend manufactured by Bunge North America Inc., was found to contain milk that was not listed on the packaging label.
Class II Recall Indicates Moderate Health Risk
The risk reclassification, issued on Wednesday, July 30, places the product under the FDA’s second-highest warning level. According to the FDA, a Class II recall involves “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
While no illnesses or allergic reactions have been reported so far, the undeclared presence of milk poses a potential health hazard to people with dairy allergies or lactose intolerance.
Initial Voluntary Recall Announced in Mid-July
The recall began as a voluntary measure by Bunge on July 14, when the company announced it was pulling approximately 64,800 pounds, or 1,800 cases, of its one-pound butter blocks from shelves. The recalled butter was packed in white paperboard cases, each containing 36 one-pound blocks.
The affected products carry the lot code 5064036503 and were shipped to 12 distribution centers across the United States and one in the Dominican Republic.
Why the Undeclared Milk Is a Serious Concern
Milk is one of the nine major food allergens identified by the FDA, alongside eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, soybeans, and sesame. The FDA mandates clear labeling of such allergens because exposure, even in small amounts, can cause a range of reactions, from mild discomfort to life-threatening symptoms.
Food-related allergic reactions may include hives, facial swelling, vomiting, coughing, and skin irritation. More severe responses can result in anaphylaxis, a rapid-onset, whole-body allergic reaction that may lead to shock and, in extreme cases, death.
According to the Mayo Clinic, anaphylaxis occurs when the immune system floods the body with chemicals in response to an allergen. This can cause a sudden drop in blood pressure, narrowing of the airways, and potential organ failure if not treated immediately.
FDA Reiterates Importance of Allergen Labeling
In light of the recall, the FDA has emphasized the importance of allergen labeling and said it continues to enforce regulations requiring companies to clearly list all ingredients and potential allergens on packaging.
“More specific labeling requirements exist for foods that can cause allergies or other hypersensitivity reactions,” the agency stated. “These rules are designed to prevent accidental consumption of allergens and to protect consumers with dietary restrictions.”
The FDA also advised that anyone who experiences symptoms of an allergic reaction after consuming the recalled butter should “stop eating the food immediately, evaluate the need to use emergency medication (such as epinephrine), and seek medical attention.”
Company Yet to Comment
As of August 2, Bunge North America has not issued an updated public statement in response to the FDA’s reclassification and did not respond to a request for comment.
Food Safety Under Scrutiny Amid Other Recent Recalls
This butter recall follows a string of other high-profile food safety incidents this year. In recent weeks, more than 110,000 cases of popular chocolate ice cream bars were recalled across 23 states. Target-branded baby food was also pulled from shelves for containing “elevated levels of lead.”
What Is Jess's Rule? How A Missed Cancer Diagnosis Led To A New Rule For General Practitioners

(Credit-Andrea Brady)
GPs in England are being urged to adopt a “Jess’s Rule”, an initiative that urges doctors to rethink their treatment if, even after three patient appointments, the patient is still experiencing the same undiagnosed symptoms. This new initiative, called Jess's Rule, is being rolled out across the NHS to help prevent avoidable deaths by catching serious illnesses earlier, especially in young people. The rule is not a law, but a strong directive designed to make a more thorough diagnostic process standard practice nationwide.
Who Is Jess from Jess’s Rule?
While this new rule may help many people, it is a direct result of the tragic story of Jessica Brady. At just 27, she passed away from advanced stage 4 cancer in 2020. Before her diagnosis, she had more than twenty appointments with her GP practice over five months, all while her symptoms were getting progressively worse. Despite her persistence, her condition was repeatedly misdiagnosed or dismissed.
Ultimately, her family had to seek private care, and only then was she diagnosed with a type of cancer so advanced that no treatment was possible. She died just three weeks after her diagnosis. Jessica's story highlights the critical need for a system that ensures a patient's concerns are taken seriously, especially when initial treatments or diagnoses aren't working.
Who Is At Risk For Less Cancer Diagnoses?
A big aspect of Jess's Rule is its focus on reducing health inequalities. Studies have shown that younger patients and those from minority backgrounds often face delays in getting a proper diagnosis. One of the reasons given was that their symptoms might not fit the typical patterns seen in older or white patients, which then leads to their concerns being dismissed.
According to Gov.UK, a report found that half of all 16 to 24-year-olds with cancer needed three or more appointments to get a diagnosis. Jess's Rule is made to combat this problem by focusing on the fact that a patient's age or background should not be a reason to overlook a potential serious illness.
The NHS hopes that this rule, which is urged to be followed across the nation, will help ensure everyone receives the same high standard of care. This initiative is a tribute to Jessica's legacy and her family's tireless efforts to prevent other families from enduring similar grief.
How Does Jess’s Rule Work?
Jess's Rule isn't a strict law, but it's a guide for medical professionals. When a patient's symptoms don't improve after three appointments, the rule suggests that the GP should take further action. This step might include moving from phone consultations to a face-to-face appointment, conducting a more in-depth physical examination, or ordering additional tests.
It also encourages doctors to review the patient's records carefully, consult with a colleague for a second opinion, or refer the patient to a specialist. The aim is to formalize what many good doctors already do: if something isn't right, don't give up.
According to Health and Social Care Secretary Wes Streeting, Jessica's death was a "preventable and unnecessary tragedy," and Jess's Rule will ensure that every patient receives "thorough, compassionate, and safe care." The initiative is a collaborative effort between the Royal College of General Practitioners (RCGP) and NHS England.
What Is Leucovorin? The Drug Trump Suggests As A Treatment For Autism?
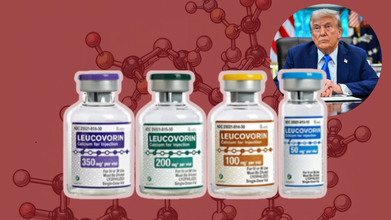
Credits: AP, TradeIndia
US President Donald Trump held a press conference to announce the connection between the use of Tylenol, a popular pain and fever relief medicine during pregnancy and autism. Trump in the press conference also suggested Leucovorin, as a potential treatment for autism symptom.
Read: Tylenol And Autism: Trump Announces Link Between The Two, Science Does Not Back Him
What Is Leucovorin?
It is known as folinic acid, a form of folate, also an essential B vitamin or B9, which has been approved by the US Food and Drug Administration (FDA) for counteracting the toxic effects of certain cancer drugs. It can counteract methotrexate that blocks body's use of folate.
The drug is also used to enhance the effects of chemotherapy drugs and to treat specific types of anemia.
How Is Leucovorin Administered? Who Makes Leucovorin?
The drug can be administered orally or intravenously. While it is manufactured by dozens of companies, notes US FDA, on Monday itself, FDA published a notice to the Federal Register ahead of Trump's press conference to approve a version of Leucovorin manufactured by GlaxoSmithKline or GSK.
GSK had previously withdrawn from FDA's consideration when it stopped making the drug. However, as per the FDA notice, "The FDA is working with GSK, the innovator of Wellcovorin (leucovorin calcium), on a process to include the essential scientific information needed for the safe and effective use of these drug products for adults and pediatric patients with CFD (cerebral folate deficiency)."
Is Leucovorin Effective For Autism?
As per the FDA notice, leucovorin calcium, branded as Wellcovorin by GSK is sent for approval for patients with cerebral folate deficiency, which is a neurological condition that affects folate, an essential vitamin for brain health, transport into the brain.
The FDA notice mentions that individuals with CFD have been "observed to have developmental delays with autistic features".
FDA Commissioner Marty Makary, MF, MPH said that in over two decades, the US has "witnessed a tragic four-fold increase in autism". He further noted: "Children are suffering and deserve access to potential treatments that have shown promise. We are using gold standard science and common sense to deliver for the American people.”
George Tidmarsh, MD, PhD, Director of the FDA's Center for Drug Evaluation and Research said that FDA is collaborating with GSK to broaden the existing Wellcovorin label and is committed to "finding and treating the root causes of autism".
Read: Trump's Claim On Linking Tylenol And Autism Is Unscientific, According To Doctors
Why Leucovorin Is Considered To Treat Autism?
Doctors have been using leucovorin off-label for autism, repurposing the drug beyond its original approval, reported Reuters.
Research from SUNY Downstate Medical Center and others suggests that as many as three-quarters of children with autism may have genetic variations or autoimmune issues that interfere with folate processing in the brain.
Smaller studies have linked these problems to more severe autism symptoms and found that leucovorin treatment may help improve speech, social interaction, and irritability. Still, the Autism Science Foundation cautions that the research is in its early stages, and more evidence is needed before firm conclusions can be drawn.
Read: Trump Suggests Changes In Childhood Vaccines, Says It Is Based On What He Feels
A 2016 study published in Molecular Psychiatry, which was a randomized controlled trial suggested that this specialized form of folate can improve communication and language skills in some children with Autism Spectrum Disorder (ASD).
A June 2020 study published in the Seminars in Pediatric Neurology suggests folate (vitamin B9) plays a vital role in brain development and function, helping with DNA synthesis, methylation, and neurotransmitter production. Some children with autism struggle with folate metabolism, particularly in transporting folate into the brain, a condition known as cerebral folate deficiency.
Another 2020 study published in October in Seminars in Pediatric Neurology suggests that in many cases, the immune system produces antibodies that target the folate receptor alpha (FRα) on brain cells. These folate receptor autoantibodies (FRAA) block folate entry into the brain, resulting in low brain folate levels even when blood levels are normal. While only about 5–10% of the general population carries these antibodies, research suggests that 50–70% of children with autism may have them. This disruption is thought to significantly affect speech and cognitive development.
However, Dr. David Mandell, a professor of psychiatry and autism expert at the University of Pennsylvania, told Reuters that leucovorin might well be a possible treatment for some children with autism, "but the evidence we have supporting it... is really, really weak."
Trump's Claim On Linking Tylenol And Autism Is Unscientific, According To Doctors

Credits: AP and Kenvue
The Monday announcement by President Trump on Tylenol and its link with autism has sparked a debate among the medical field. Experts have claimed that the link suggested is unfound in science. Australia's peak body for obstetricians and gynaecologists now fear that pregnant women will not take paracetamol when they need it and suffer harm from unmanaged fever.
Trump suggested that women should "fight like hell" and not take paracetamol, which is branded as Tylenol in the US, as it heightens the risk of autism when it is used by pregnant women. He further said that if women continue to take the medicine, "that would mean you can't tough it out, so that's up to you and your doctor. I just want to say it is like it is: don't take Tylenol. Don't take it. Fight like hell not to take it."
However, as far as science and facts are concerned, the pain medicine is widely considered a safe option to treat pain and fever even during pregnancy.
As a result of this, Australia's medicine regulator, the Therapeutic Goods Administration (TGA), re-confirmed the safety of this drug use during pregnancy.
Also Read: Tylenol And Autism: Trump Announces Link Between The Two, Science Does Not Back Him
What Is The Australian Medical Body Saying About Tylenol Use?
The Royal Australian and New Zeeland College of Obstetricians and Gynaecologists (RANZCOG) warned that Trump’s comments could lead pregnant women to avoid paracetamol and suffer harm from unmanaged fever.
Dr Elisha Broom, a counsellor and spokesperson for RANZCOG, as reported by Guardian said, evidence supports the safety of paracetamol in pregnancy when used correctly. Broom further noted that unmanaged fever is a known risk to pregnancy, unlike paracetamol.
“It’s not a no-harm scenario when women are fearful to take what we know are safe medications,” said Broom.
Broom is a maternal fetal medicine sub-specialist in Queensland and said that many obstetricians are expecting questions from their patients after Trump's announcement and would welcome them.
Read: What Is Leucovorin - The Drug Trump Suggests As A Treatment For Autism?
Is There A Link Between Fever Medicines And Babies?
Broom said, "We know that actually there is a link between fever and impacts on babies – not neurodivergence – but complications in pregnancies that result from unmanaged fever."
She further noted that "It is not a no-harm scenario where women are fearful to take what we know are safe over-the-counter medications to relieve pain and fever in pregnancy."
The reason why this announcement could harm women more than protecting them is because medicines like Nurofen or ibuprofen is already not recommended because it does not have the same safety profile, suggests Broom. Thus, this leaves women without any options but to cope with pain and unmanaged fever.
The TGA also noted that "paracetamol remains Pregnancy Category A in Australia, meaning that it is considered safe for use in pregnancy”.
It said: “The TGA maintains robust post-market safety surveillance and pharmacovigilance processes for all medicines registered in Australia, including paracetamol.”
Read: Trump Suggests Changes In Childhood Vaccines, Says It Is Based On What He Feels
What Do Other Medical Boards Say About This Announcement?
Australian Medical Association (AMA) president, Danielle McMullen stressed that studies showing a “possible association” between paracetamol and autism do not prove causation. She said autism is far more likely to be driven by genetic factors.
The American College of Obstetricians and Gynecologists (ACOG) also called Trump's announcement "irresponsible" and "highly unsettling". They emphasized acetaminophen (Tylenol) remains the safest painkiller during pregnancy.
© 2024 Bennett, Coleman & Company Limited

