- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
People With MS Can Benefit From This Cancer Drug, Finds Study

Credits: Canva
A new clinical trial, published in The New England Journal of Medicine found that an experimental drug, named tolebrutinib could help delay the progression of disability in patients with non-relapsing secondary progressive multiple sclerosis (SPMS).
What Is SPMS?
This is a form of multiple sclerosis where symptoms steadily worsen over time. It follows an initial period of relapsing and remitting MS. Which means, overtime, the patients with SPMS experience fewer relapses, but it steadily keeps getting worse and leads to neurological decline. As of now, there are no FDA-approved treatments for non-relapsing form of SPMS, which makes this trial's results significant.
As per the National Multiple Sclerosis Society, MS is an unpredictable disease of the central nervous system that disrupts the flow of information to and from the brain. There are 4 disease courses in MS, Secondary progressive multiple sclerosis (SPMS) follows the initial course of relapsing-remitting multiple sclerosis (RRMS).
How Was The Study Conducted?
For this study, the researchers enrolled over 1,100 MS patients who were between the ages of 18 to 60 from 264 cities in 31 countries. The participants were randomly assigned to receive either a daily dose of tolebrutinib or a placebo.
What Is Tolebrutinib?
It is a tyrosine kinase inhibitor, which means, it is a class of drugs that was originally designed to treat cancers like lymphoma. It works by blocking enzymes that could promote inflammation and abnormal cell activities.
The study found that after six months, 22.8% of patients taking tolebrutinib had progressed in their MS-related disability, compared to 30.8% in the placebo group. This marks a 31% reduction in disability progression. The drug also showed benefits in several other areas:
Improved Disability Scores: 8.6% of those who were administered with tolebrutinib showed improvement in disability, nearly double the 4.5% in the placebo group
Better Mobility: Patients who took the drug experienced a sustained 20% improvement in a timed 25-foot walk test after three months
Reduced Disease Activity: The MRI scans too showed fewer new or enlarging brain and spinal cord lesions for those who consumed tolebrutinib
Are There Any Safety Concerns?
Despite its benefits, tolebrutinib comes with potential risks—most notably liver injury. Elevated liver enzyme levels, which can signal liver damage, occurred in 4% of patients taking the drug, compared to 1.6% in the placebo group. While most cases were manageable, about 1 in 200 patients showed severe elevation in liver enzymes, requiring the drug to be discontinued.
“If approved, the drug will likely require intensive monitoring of liver function during the first three months,” said Dr. Robert Fox, the lead researcher and neurologist at the Cleveland Clinic. He emphasized the importance of early detection of liver-related issues to ensure safe treatment.
Tolebrutinib may represent a breakthrough for patients with non-relapsing SPMS, offering the first targeted treatment for slowing disability in the later stages of the disease. “It offers the potential for a new class of medications for MS,” said Dr. Fox, “which remains the leading cause of non-traumatic disability in young adults.”
Pending FDA approval, tolebrutinib could offer hope to thousands living with progressive MS, giving them not only more time but a better quality of life.
Kennedy Accused Of Destabilizing Vaccine Policy By Ousted CDC Experts

Credits: Getty Images
RFK Jr ousted all 17 experts from the vaccine advisory panel. As a counter, all the 17 experts have published a joint essay criticizing the US Health Secretary Robert F Kennedy Jr. for making abrupt and "destabilizing decisions". They said that this could jeopardize the nation's immunization strategy and increase the risk of preventable disease outbreaks.
The former panelists, ousted last week, voiced their concerns in an article published Monday in the Journal of the American Medical Association (JAMA). Their essay warns that Kennedy's actions—including disbanding the existing panel, dismissing long-serving CDC staff, and appointing controversial new members—could have serious consequences for public health.
“We are deeply concerned that these destabilizing decisions, made without clear rationale, may roll back the achievements of U.S. immunization policy, impact people’s access to lifesaving vaccines, and ultimately put U.S. families at risk of dangerous and preventable illnesses,” the authors wrote.
A Sudden Shift In Key Advisory Panel
It was only last week when Kennedy announced the "retirement" of the entire panel. This was the Advisory Committee on Immunization Practices (ACIP), the panel responsible for shaping U.S. vaccine policy. He also removed Dr. Melinda Wharton, a senior Centers for Disease Control and Prevention (CDC) official who coordinated the committee’s meetings.
Just two days after dissolving the panel, Kennedy appointed eight new members. Among them are a scientist critical of COVID-19 vaccines, a vocal opponent of pandemic lockdowns, and a member of an organization widely labeled as a key source of vaccine misinformation.
The new committee is scheduled to meet next week, though no agenda has been made public. A federal notice indicated that votes are expected on recommendations for vaccines against influenza, COVID-19, HPV, RSV, and meningococcal bacteria.
CDC Departures Raise Alarm
In addition to Wharton’s removal, several key CDC immunization staffers have either been reassigned or have resigned. Notably, Dr. Lakshmi Panagiotakopoulos left the agency earlier this month after 12 years, informing members of a COVID-19 vaccine workgroup of her decision.
Her resignation came after Kennedy revoked CDC’s recommendation for COVID-19 vaccination for healthy children and pregnant women—without consulting the advisory panel.
“My career in public health and vaccinology started with a deep-seated desire to help the most vulnerable members of our population, and that is not something I am able to continue doing in this role,” she wrote in a message viewed by the Associated Press.
The former ACIP members emphasized that the loss of experienced CDC staff would hinder the new advisers’ ability to make evidence-based recommendations swiftly and effectively.
Kennedy’s Criticisms and Questions of Credibility
A spokesperson for the U.S. Department of Health and Human Services declined to address the essay directly but referred to Kennedy’s previous comments accusing the advisory committee of being too close to vaccine manufacturers and rubber-stamping recommendations.
Kennedy, who long voiced anti-vaccine sentiments before being appointed as health secretary, has questioned the integrity of the ACIP’s review process. Though committee members are required to disclose ties to vaccine companies and recuse themselves in cases of conflict of interest, Kennedy has dismissed these safeguards as inadequate.
Founded in 1964, the ACIP provides guidance to the CDC director on how FDA-approved vaccines should be administered. Its recommendations are widely followed by medical professionals and underpin the nation’s immunization programs.
The abrupt dismissal of the entire panel, according to the former members, not only disrupts a well-established public health process but also undermines trust and efficiency at a time when evidence-based decision-making is critical.
Spike In ‘Gas Station Heroin’ Use Sparks National Health Warning

Credits: Canva and photo by FDA
US health officials are raising serious concerns about 'Gas Station Heroin', a substance called tianeptine. This has opioid-like-effects and the availability and accessibility is only increasing.
It is also sold as over-the-counter supplements at gas stations, smoke shops, and convenience store. The drug is also marketed as Zaza, Tianaa, Pegasus, and TD Red.
Though used as an antidepressant in some other countries, tianeptine is not approved for medical use in the United States. Experts warn that its effects can mimic those of opioids, including addiction and withdrawal symptoms, even though it is not officially classified as one.
Also Read: Lethal Fungus Is Spreading Across Many US States, Why Is It Concerning?
What Is Tianeptine?
A 2023 study published in journal Pain and Therapy, notes: "Tianeptine is an antidepressant drug approved for the treatment of major depressive disorder in countries other than the US. It is classified as an atypical tricyclic antidepressant and has shown potential benefits in addressing anxiety and irritable bowel disease. However, it is important to note that tianeptine is not approved for any use by the United States Federal Drug Administration (FDA). Despite its lack of approval by the FDA, tianeptine has been distributed online and at small retail locations."
The term “gas station drugs” refers to a wide range of substances typically available for purchase from gas stations, corner stores, bodegas, mini marts, smoke shops, and the Internet. These substances may be produced commercially by drug manufacturers or in clandestine laboratories to mimic the effects of more well-known illicit/controlled substances such as marijuana, cocaine, opioids, etc.
The study also notes that misuse of tianeptine can lead to euphoric, opioid-like highs with the potential for chronic users to develop dependence and tolerance. Overdose and use in suicide attempts have also been documented.
Its popularity stems from its ability to bind to the same brain receptors as opioids, which can create feelings of euphoria, calmness, and even pain relief. “It’s kind of this grey area of consumer products, or supplements, where the contents are not regulated or tested the way they would be with a medication,” explained Dr. Diane Calello of the New Jersey Poison Information and Education System. “You never quite know what’s in that bottle.”
Also Read: Eye Signs That Warn of Heart Disease: What Your Eyes Reveal About Heart Health
FDA Warns Against Its Easy Accessibility
In response to rising reports of adverse effects, the FDA issued a formal warning last month, calling tianeptine "a dangerous and growing health trend." The agency urged retailers to stop selling these products and asked consumers to avoid purchasing or using them altogether.
Poison control centers have seen a sharp rise in tianeptine-related incidents. The FDA noted that many of these cases involve young people, and the symptoms often require emergency medical attention. Reported side effects include seizures, low blood pressure, rapid heartbeat, and mental distress. In a review of 20 recent cases, more than half of the patients had to be admitted to intensive care units.
The Alabama Case Study
The state of Alabama serves as a stark example of the risks associated with tianeptine. Between 2018 and 2021, poison control calls related to the drug spiked by over 1,400%. However, after the state imposed strict regulations and bans, the number of such incidents saw a notable decline. This indicates that legal restrictions can play a significant role in curbing misuse.
Health professionals are urging federal and state authorities to take faster and stronger action before tianeptine use becomes a full-blown public health crisis. Some compare the current trajectory of the drug to the early days of the opioid epidemic, which caught the country off guard and led to long-term consequences.
Lethal Fungus Is Spreading Across Many US States, Why Is It Concerning?
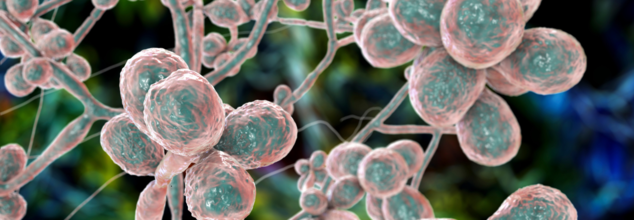
Credits: Canva
A dangerous airborne fungus that has the potential to rot human tissue from the inside is spreading at a considerably increasing rate across the United States. It has prompted concerns among the medical professionals and public health authorities. Aspergillus fumigatus. This is said to be the culprit behind this surge and poses a risk to especially those whose immune system had been compromised. The cases are becoming harder to treat due to widespread drug resistance and climate-linked proliferation.
The World Health Organization (WHO) has classified Aspergillus fumigatus as a “critical priority” due to its high mortality rate and growing resistance to existing treatments.
Silent Invader
It is called a silent invader for a reason. Once you inhale, the fungus can cause aspergillosis. This is a serious lung infection. The spores are microscopic, which mean hat people can breathe them in unknowingly. For patients who are already vulnerable medically, for instance, those battling cancer, asthma, HIV or recovering from organ transplants, the infection can lead to chronic lung disease, systemic organ failure, or even death. If invasive, it can also spread to your brain, hear, and kidneys.
Despite its potentially fatal consequences, aspergillosis is not classified as a reportable disease in the United States. This means infections are not systematically tracked, leaving health officials with limited data and making it harder to trace or contain outbreaks.
Infections On The Rise
Between 2000 to 2013, hospitalizations have increased in the case of invasive aspergillosis in the US. The rate is reported by a 3% increased, annually. In 2014, the infection led to 15,000 hospital stays, with a cost up to $1.2 billion.
As the US News notes, postmortem examinations in intensive care units suggest that aspergillosis is among the top four infections most likely to be fatal.
While about 400,000 Americans may be living with chronic pulmonary aspergillosis — the long-term form of the disease — invasive cases, though less frequent, are significantly deadlier. Survival rates drop sharply: just 59% of organ transplant patients and only 25% of stem cell transplant patients live beyond a year after infection.
What Is Causing Its Spread?
The fungus thrives in hot, damp, and humid environment. They can also endure up to 120 degree Fahrenheit. This is especially true in compost or agricultural waste. States like Florida, Texas, Georgia, Louisiana, and California — with their humid climates and farming activity — are seeing some of the highest exposure levels. Cities such as New York, Houston, and Los Angeles are also at risk due to overcrowding, aging buildings, and limited ventilation.
According to a University of Manchester study, if fossil fuel use continues at current levels, Aspergillus fumigatus could expand its range by over 75% by 2100 — putting millions more at risk across the southern US.
What is making it even more difficult to control is the increasing drug resistance. Azole drugs — commonly used to treat human fungal infections — are also applied to protect crops. This dual-use, experts say, may be fueling cross-environmental resistance, with drug-resistant spores jumping from soil to humans.
A study published in Applied and Environmental Microbiology found resistant fungal strains in agricultural soil across at least seven US states. Some strains are resistant to multiple standard antifungals, making treatment increasingly difficult.
WHO has also urged pharmaceutical companies to invest in new antifungals, including children in drug trials, and enhance diagnostic and treatment protocols globally.
Preventive Measures
As of now, doctors recommend to avoid contact with soil, mold, and gardening. It is important to wear protective masks when in dusty areas, and ensure good air filtration at home.
© 2024 Bennett, Coleman & Company Limited

